Processing method of aconitine hydroxyl reduction structure converted aconitine traditional Chinese medicine decoction pieces
A technology of aconitine hydroxyl group and Chinese herbal decoction pieces, which is applied in medical formulas, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as harsh reduction reaction conditions, complex reaction products, and weak selectivity. Simple, single reaction product, and easy-to-control conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
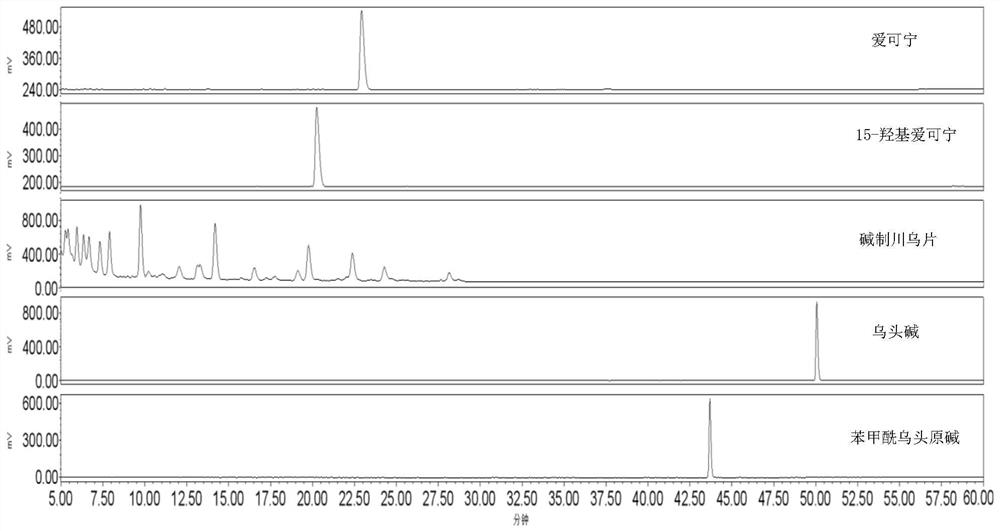
[0033] Take 8kg of sodium carbonate, put it in a bucket, add 40L of deionized water, stir to dissolve, and get lye; take 10kg of raw aconitum slices, put them in the above-mentioned 20% sodium carbonate solution, soak for 12hr, take out the moistened decoction pieces, and drain them. Let it moisten for 3 hours, put it in a steamer, steam for 60 minutes under normal pressure, steam through the heart, take it out, and dry it under reduced pressure at 60°C to obtain concentrated alkali-made Aconitum slices. Benzoyl aconitine, aconitine reference substance, and the methanol solution of aconine and 15-hydroxyecgonine prepared separately were used as controls, and the methanol ultrasonic extract of alkali-made aconitum tablets was analyzed by HPLC. Chromatographic conditions: C18 filler, 250mm*4.5mm column, flow rate 1ml / min, ELSD detector (gas flow rate 2.8mL / min, drift tube temperature 110°C), chromatographic gradient is shown in Table 1, and the chromatogram is as follows figure ...
Embodiment 2
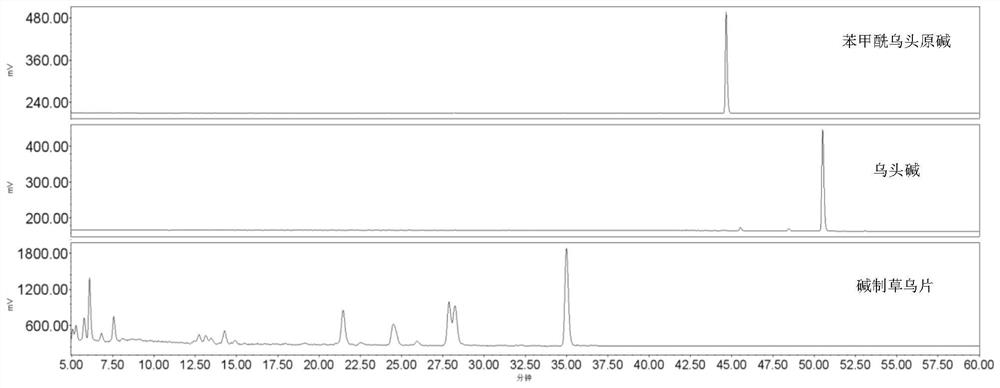
[0038] Take 7kg of sodium carbonate and 1kg of sodium hydroxide in a bucket, add 40L of deionized water, stir and dissolve to obtain lye; take 10kg of Aconitum aconitum slices, put them in the above-mentioned 20% alkali solution, soak for 12hr, take out the moistened The decoction pieces were moistened for 3 hours, placed in a steamer, steamed at normal pressure for 60 minutes, steamed through the heart, taken out, and dried at 60°C under reduced pressure to obtain Aconitum aconitum tablets made with concentrated alkali. The methanol solution of benzoyl aconitine and aconitine reference substance was used as the control, and the methanol ultrasonic extract of Aconitum aconitum prepared by alkali was injected and analyzed by HPLC. The chromatographic conditions are as in Example 1, and the results are as follows figure 2 .
[0039] The results showed that aconitine and benzoylaconitine were not detected in Concentrated Alkali-Prepared Aconitum Tablets by HPLC-ELSD method.
Embodiment 3
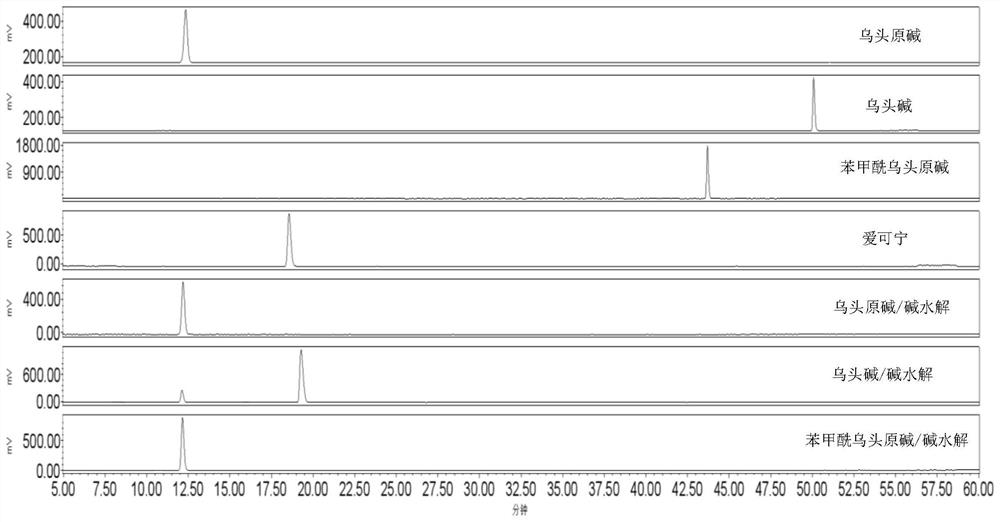
[0041] Take 5 kg of Aconitum slices made from medium-concentrated alkali, put them in a frying pot, add 50 L of water, heat and decoct for 60 minutes, and filter out the medicinal liquid; add 35 L of water to the dregs of the medicine in the pot, heat and decoct for 30 minutes, and filter out the medicinal liquid , combine the filtrates, let stand for 6 hours, filter the supernatant, cool to 40-50°C, and filter; take 2.5L pretreated AB-8 large-pore resin, put it into a chromatography column, wash with water to remove air bubbles, and put the filtrate on the resin column , the flow rate of the liquid medicine is 250ml / min. After the liquid medicine is exhausted, use 25L of deionized water to elute to remove inorganic substances, and then use 10L of 15% ethanol to elute; 10L of 15% ethanol solution was used for elution, the eluate was collected, the solvent was recovered, and dried under reduced pressure to obtain total aconitine of aconitum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com