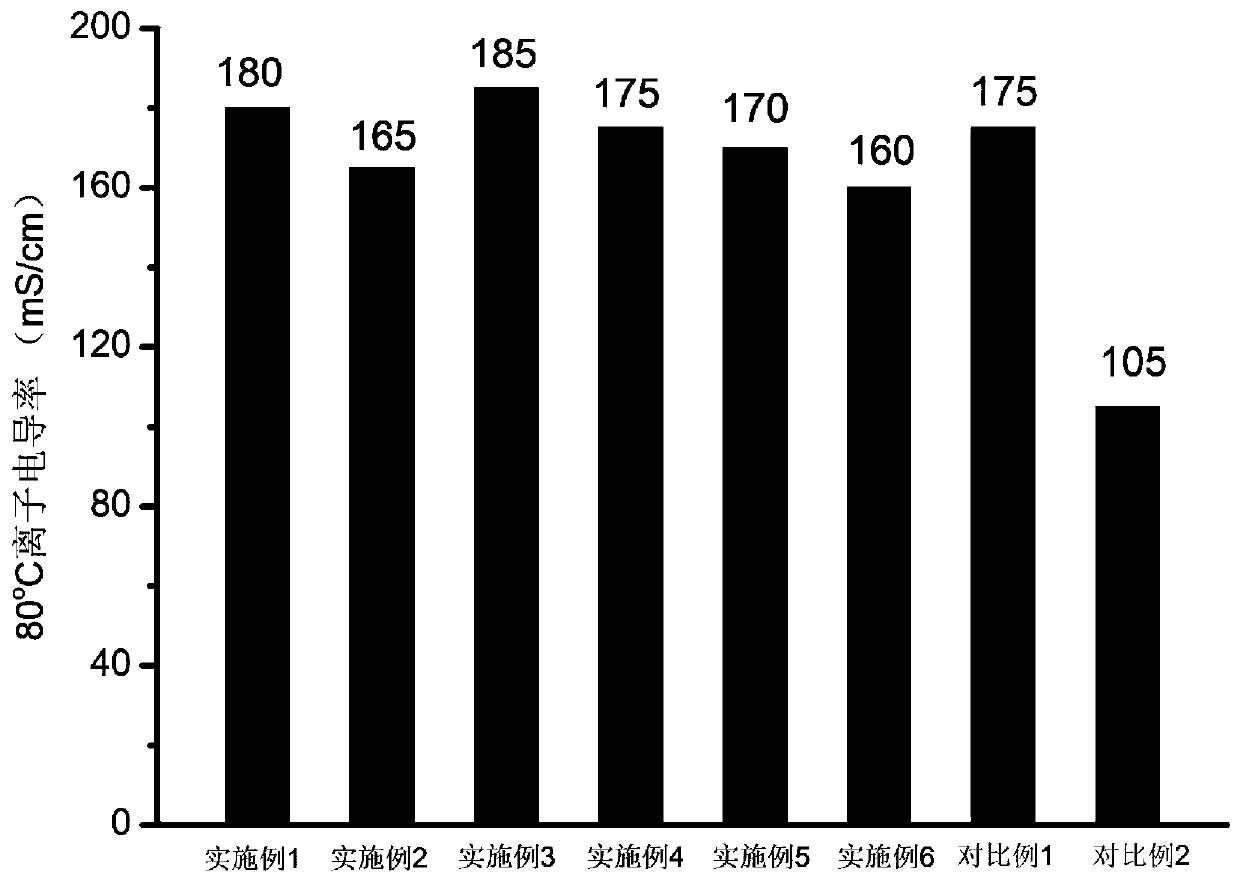
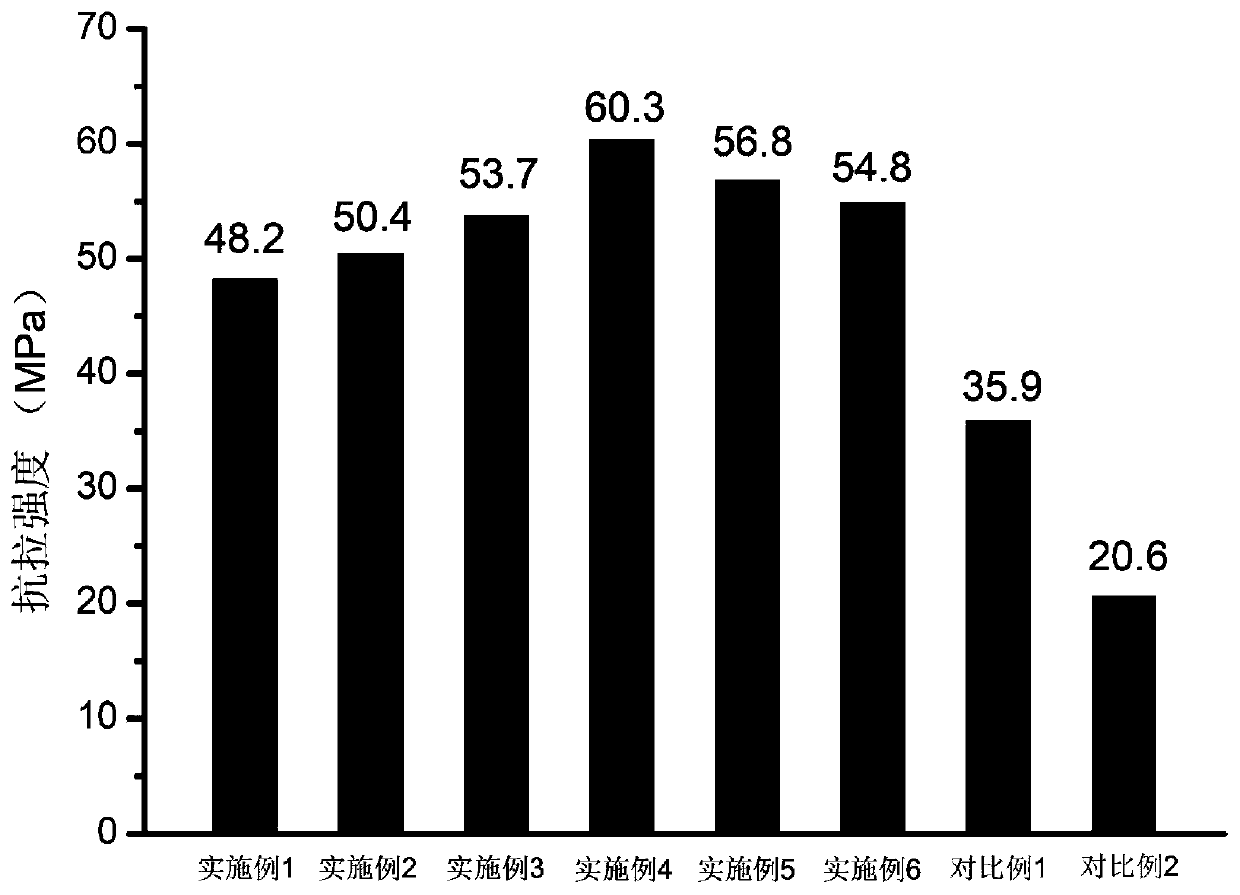
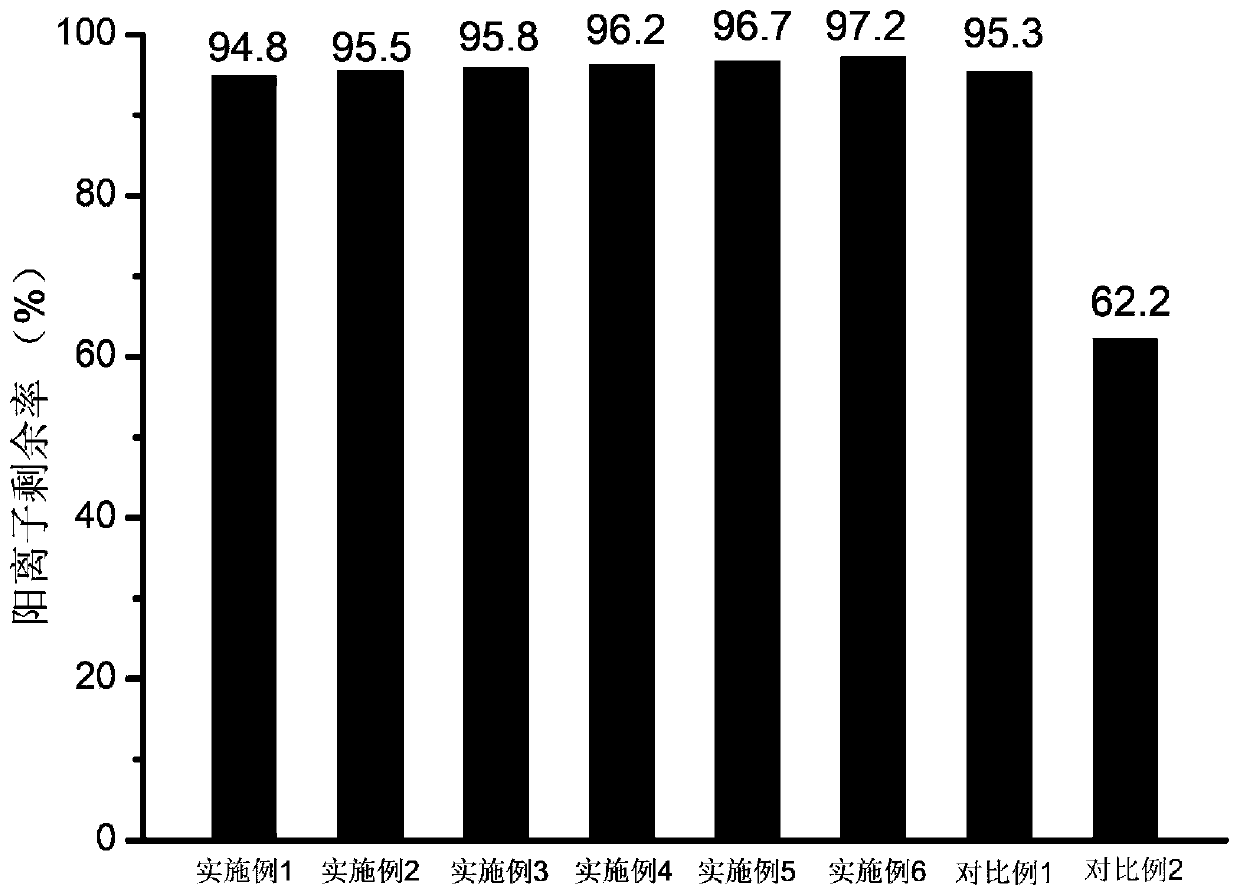
Piperidine tertiary amine group-containing polymer, anion exchange polymer and preparation method and application thereof
A polymer, pyridine tertiary amine technology, applied in the direction of anion exchange, ion exchange, chemical instruments and methods, etc., can solve the problem that the mechanical strength needs to be further improved, and achieve excellent chemical stability, high ionic conductivity, and excellent mechanical strength. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] This embodiment provides an anion exchange polymer whose structure is as follows:
[0105]
[0106] The preparation route is as follows:
[0107]
[0108] (1) Synthesis of polymers containing piperidine tertiary amine groups
[0109] Weigh 7.98 g (48.0 mmol) of fluorene into a 100 mL three-necked flask, add 5.43 g (48.0 mmol) of N-methyl-4-piperidone, and add 15 mL of dichloromethane to dissolve the reactant. Add 50 mL of a mixed acid of trifluoromethanesulfonic acid and trifluoroacetic acid (the volume ratio of the two is 12:1) at 0°C, and react for 6 hours. Pour the viscous purple product into 1M K 2 CO 3 Soaked in the solution for 24 hours at room temperature, filtered to obtain a white solid product, thoroughly washed with deionized water and dried to obtain a polymer containing a piperidine tertiary amine group.
[0110] (2) Quaternization reaction
[0111] Weigh 10.0 g of the above intermediate polymer, add 100 mL of 1-methyl-2-pyrrolidone, completely dissolve, add 18 mL ...
Embodiment 2
[0116] This embodiment provides an anion exchange polymer whose structure is as follows:
[0117]
[0118] The preparation route is as follows:
[0119]
[0120] The preparation method is different from Example 1 only in that 7.98 g of fluorene is replaced with 15.19 g (48.0 mmol) of 9,9-spirobifluorene, and other conditions are completely the same as those of Example 1.
[0121] The number average molecular weight of the obtained anion exchange polymer was 210,000. Its NMR spectrum is as follows Figure 5 As shown, the chemical shift (δ) 2.50ppm is the DMSO solvent peak, the δ2.85ppm and δ3.35ppm correspond to the characteristic peaks of methylene H on the piperidine ring, and δ3.15ppm corresponds to the methyl group directly connected to the nitrogen atom. The characteristic peak of H, δ7.20~7.90ppm is the multiple characteristic peak of H on the benzene ring in the 9,9-spirobifluorene structural unit. There are seven kinds of H in different chemical environments.
Embodiment 3
[0123] This embodiment provides an anion exchange polymer whose structure is as follows:
[0124]
[0125] The preparation route is as follows:
[0126]
[0127] The preparation method is different from Example 1 only in that 7.98g of fluorene is replaced with 5.32g (32.0mmol) of fluorene and 2.47g (16.0mmol) of biphenyl. Compared with Example 1, the other conditions are exactly the same.
[0128] The number average molecular weight of the resulting anion exchange polymer was 185,000. Its NMR spectrum is as follows Image 6 As shown, the chemical shift (δ) 2.50ppm is the DMSO solvent peak, the δ2.85ppm and δ3.35ppm correspond to the characteristic peaks of methylene H on the piperidine ring, and δ3.15ppm corresponds to the methyl group directly connected to the nitrogen atom. The characteristic peak of H, δ3.95ppm is the characteristic peak of methylene H in the fluorene structural unit, and δ7.40~7.90ppm is the multiple characteristic peak of H on the benzene ring in the main chain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com