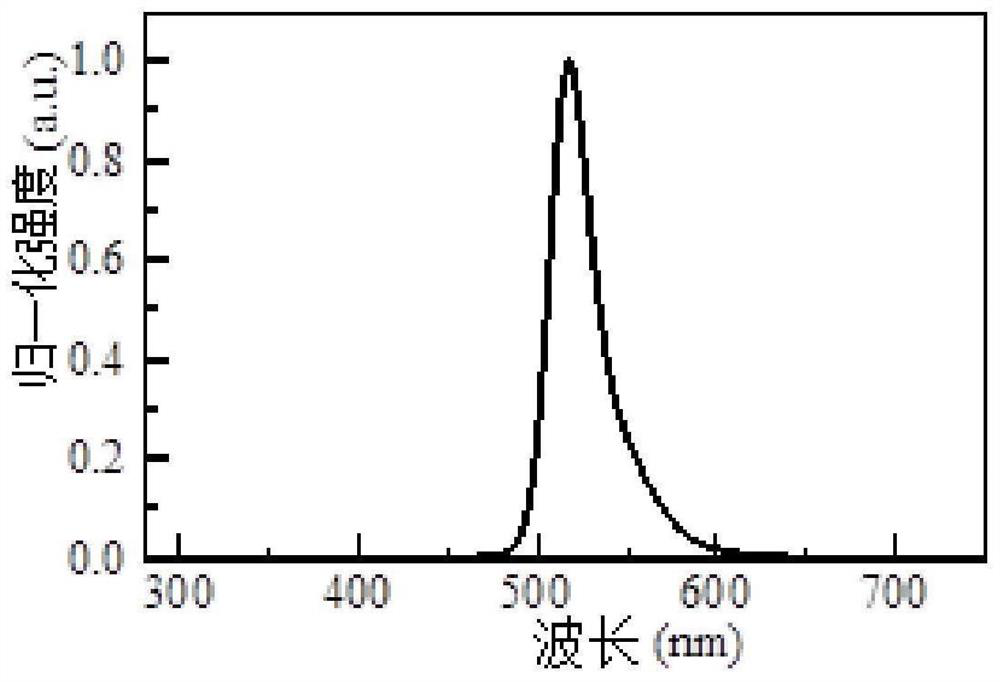
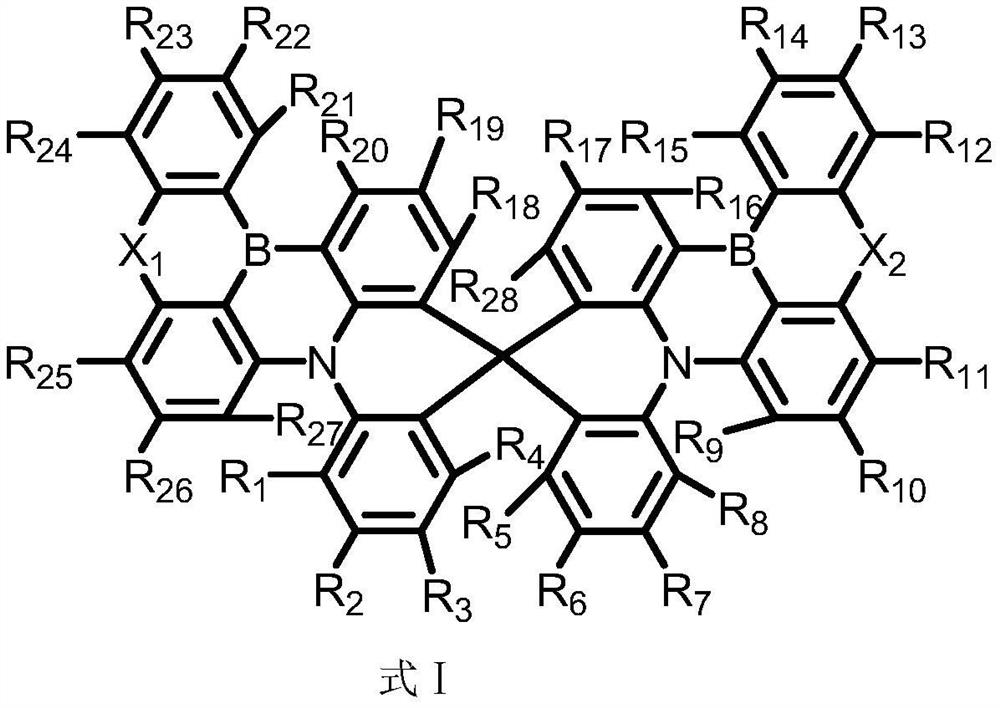
Boron-containing spiro compound and organic electroluminescent device
A spiro compound, unsubstituted technology, applied in the field of electronic materials, can solve problems such as easy aggregation, poor color purity of materials and devices with luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127]This embodiment provides a boron-containing spiro compound S-1, which has the following structure:
[0128]
[0129] The synthetic route of compound S-1 is as follows:
[0130]
[0131] The preparation method of compound S-1 specifically comprises the following steps:
[0132] 1) Synthesis of intermediate 3
[0133] Compound 1 (3.48g, 10mmol) was dissolved in 30mL of dry tetrahydrofuran, cooled to -78°C, n-BuLi (2.5mol / L, 11mmol) was added dropwise to the above solution, and the reaction was continued at this temperature for 2h; Compound 2 (2.83g, 10mmol) was dissolved in 20mL of dry THF, and added dropwise to the above mixture, continued to react at -78°C for 30min, then naturally warmed to room temperature, and reacted at room temperature for 12h, and 20mL 3mol / L of HCl was added dropwise to the above mixed solution, the reaction was continued for 12h, and the above mixed solution was poured into saturated Na 2 CO 3 solution, and then utilize dichloromethane ...
Embodiment 2
[0142] This embodiment provides a boron-containing spiro compound S-69, which has the following structure:
[0143]
[0144] The synthetic route of compound S-69 is as follows:
[0145]
[0146] The preparation method of compound S-69 specifically comprises the following steps:
[0147] 1) Synthesis of Intermediate 6
[0148] The above intermediate 4 (1.2g, 2mmol) and naphthol (577mg, 4mmol) were dissolved in 10mL N-methylpyrrolidone, cesium carbonate (2.6g, 8mmol) was added, and the temperature was raised to 170°C for 18h. After the reaction was completed, , cooled to room temperature, then using dichloromethane to extract the reaction solution, drying the organic phase with anhydrous sodium sulfate, spinning to remove the organic solvent, and separating by column chromatography to obtain white solid intermediate 6 (1.16g, 68%), MS (EI):m / z 850.2[M + ].
[0149] 2) Synthesis of target product S-69
[0150] Dissolve the above-prepared intermediate 6 (852mg, 1mmol) i...
Embodiment 3
[0152] This embodiment provides a boron-containing spiro compound S-1036, which has the following structure:
[0153]
[0154] The synthetic route of compound S-1036 is as follows:
[0155]
[0156] The preparation method of compound S-1036 specifically comprises the following steps:
[0157] 1) Synthesis of Intermediate 7
[0158] The above intermediate 4 (1.2g, 2mmol) and p-cyanophenol (476mg, 3mmol) were dissolved in 10mL of N-methylpyrrolidone, cesium carbonate (2.6g, 8mmol) was added, and the temperature was raised to 170°C for 18h. After completion, cool to room temperature, then use dichloromethane to extract the reaction solution, dry the organic phase with anhydrous sodium sulfate, spin dry to remove the organic solvent, and separate by column chromatography to obtain white solid intermediate 7 (1.12g, 70%) , MS(EI):m / z 800.1[M + ].
[0159] 2) Synthesis of target product S-1036
[0160] Dissolve the above-prepared intermediate 7 (802mg, 1mmol) in 40mL of d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com