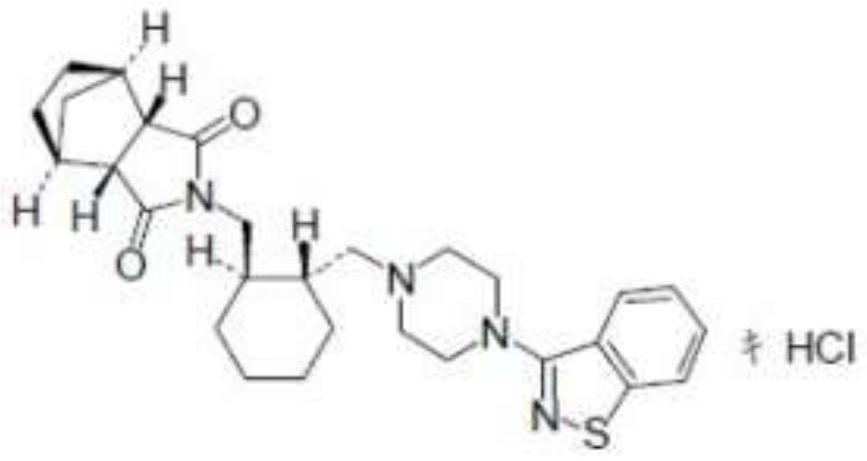
Lurasidone sublingual tablet and preparation method thereof
A technology of lurasidone and lurasidone hydrochloride, which is applied in the field of pharmaceutical preparations, can solve the problems of difficulty in drug treatment, slow disintegration speed, and long onset time, and achieve fewer types of excipients, faster disintegration speed, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, preparation lurasidone sublingual tablet
[0024] 【Prescription Composition】
[0025] components Dosage g / 1000 tablets lurasidone hydrochloride 40 Sorbitol 20 Spray Dried Mannitol 80 Crospovidone 5 Hydroxypropyl Cellulose 3 aspartame 1 Magnesium stearate 1
[0026] Wherein, the particle size range of lurasidone hydrochloride is 0.1-1 μm.
[0027] 【Preparation】
[0028] Mix the raw material lurasidone with sorbitol, crospovidone, and hydroxypropyl cellulose passed through a 100-mesh sieve, and then add spray-dried mannitol and aspartame, after mixing evenly, add magnesium stearate, Tablets are obtained as sublingual tablets.
Embodiment 2
[0029] Embodiment 2, preparation lurasidone sublingual tablet
[0030] 【Prescription Composition】
[0031] components Dosage g / 1000 tablets lurasidone hydrochloride 40 Sorbitol 40 Spray Dried Mannitol 60 Crospovidone 10 Hydroxypropyl Cellulose 2 aspartame 1 Magnesium stearate 1
[0032] Wherein, the particle size range of lurasidone hydrochloride is 0.1-1 μm.
[0033] 【Preparation】
[0034] Mix the raw material lurasidone with sorbitol, crospovidone, and hydroxypropyl cellulose passed through a 100-mesh sieve, and then add spray-dried mannitol and aspartame, after mixing evenly, add magnesium stearate, Tablets are obtained as sublingual tablets.
Embodiment 3
[0035] Embodiment 3, preparation lurasidone sublingual tablet
[0036] 【Prescription Composition】
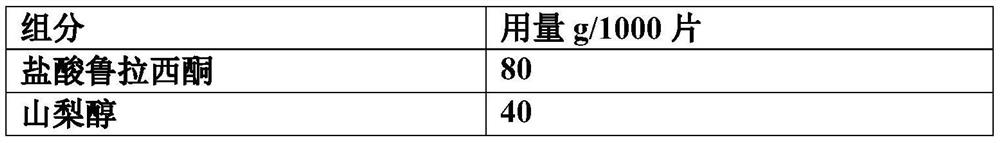
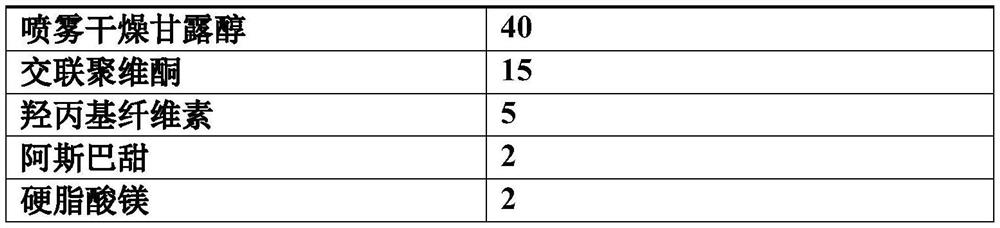
[0037] components Dosage g / 1000 tablets lurasidone hydrochloride 80 Sorbitol 20 Mannitol 80 Crospovidone 15 Hydroxypropyl Cellulose 5 aspartame 2 Magnesium stearate 2
[0038] 【Preparation】
[0039] Mix the raw material lurasidone with sorbitol, crospovidone, and hydroxypropyl cellulose passed through a 100-mesh sieve, and then add spray-dried mannitol and aspartame, after mixing evenly, add magnesium stearate, Tablets are obtained as sublingual tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com