Preparation method and application of bimetallic nano-catalyst
A bimetallic nano-catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problem that bimetallic nano-catalysts have not yet appeared.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Step 1: Add 23.28 mg Co(NO 3 ) 2 ·6H 2 O was dissolved in 1mL deionized water, stirred and dissolved, and the Co 2+ aqueous solution;
[0045] Step 2: Add 8.08 mg of ruthenium carbon to 8 mL of deionized water and stir thoroughly;
[0046] Step 3: Take the Co that was stirred evenly in Step 1 2+ Add the ruthenium-carbon dispersion in step 2 after the aqueous solution, and fully magnetically stir until uniform;
[0047] Step 4: Quickly add 1 mL of ammonia borane solution with a concentration of 1.0 mmol / mL to the solution obtained in step 3, and stir for 15-30 min until the reaction is completed;
[0048] Step 5: Wash and centrifuge the precipitate obtained in step 4, then dry the centrifuged precipitate at 60-65°C under vacuum and grind it into powder to prepare the Co-Ru / C nano catalyst.
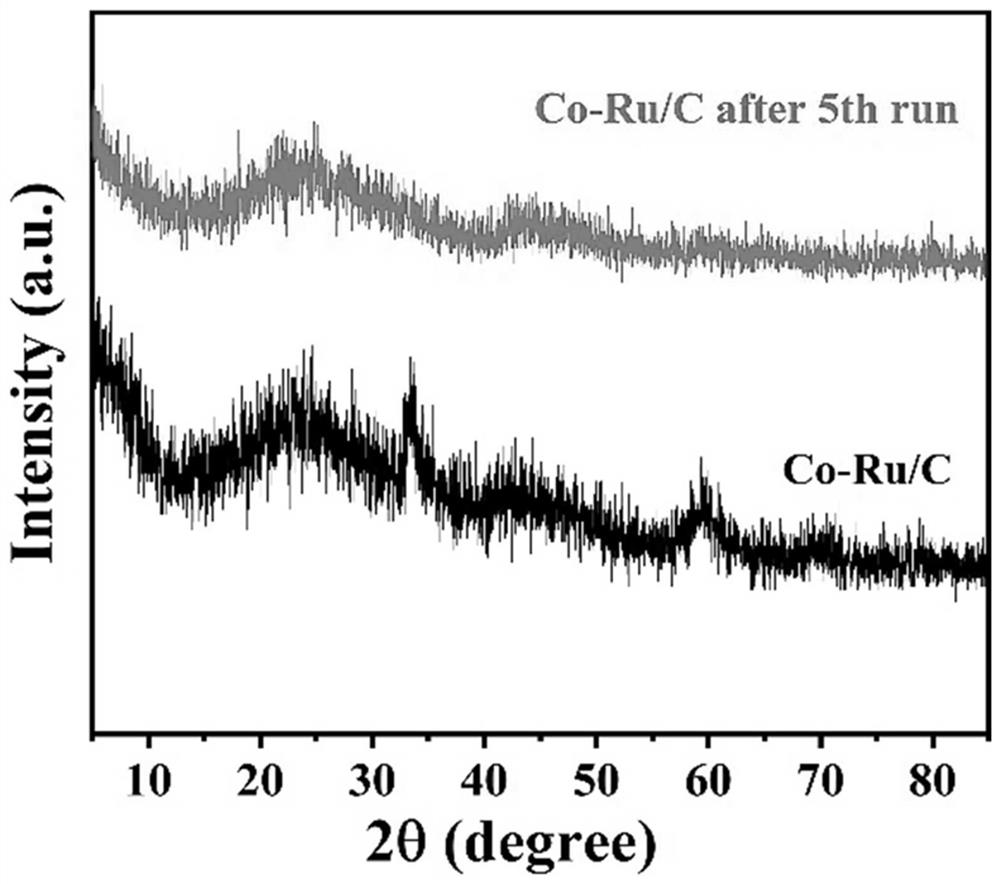
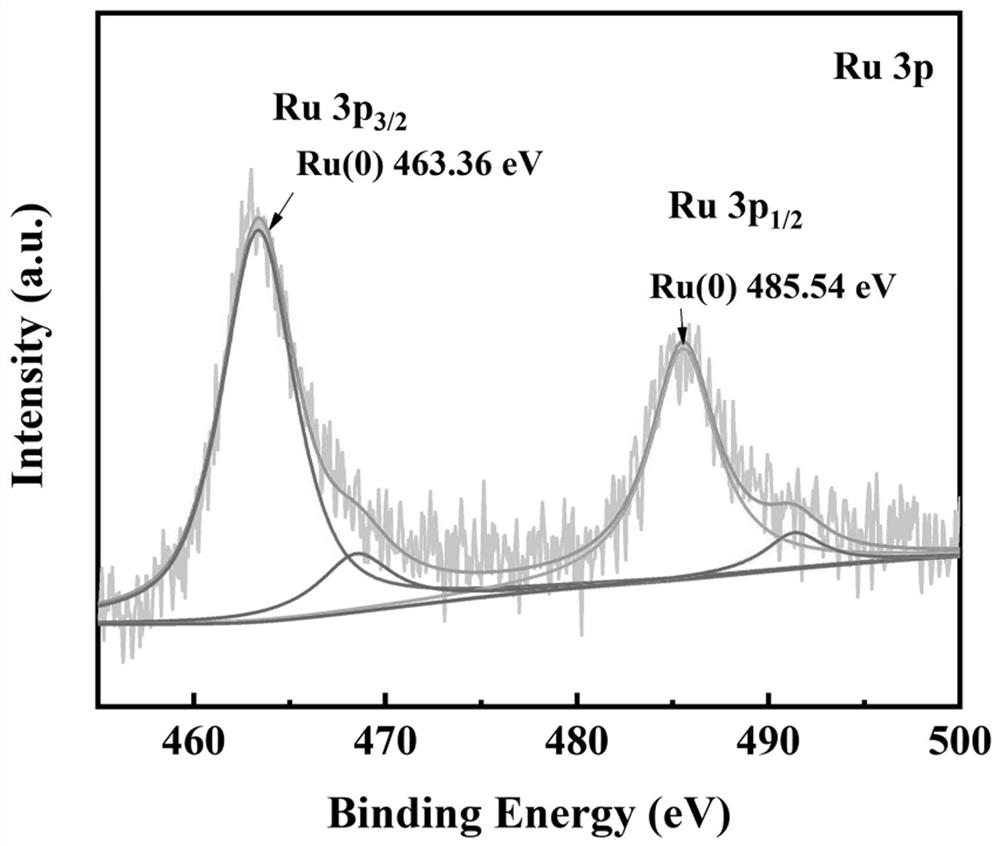
[0049] figure 1 The HRTEM diagram of the Co-Ru / C nanocatalyst prepared in Example 1 of the present invention, it can be proved from the figure that the catalyst contains cobal...
Embodiment 2
[0054] The Co-Ru / C nanocatalyst prepared according to embodiment 1 is catalytic sodium borohydride (NaBH 4 ) The specific steps of hydrolysis hydrogen production reaction are as follows:
[0055] Step 1: Dissolve 1 mmol sodium borohydride in deionized water to form a 1.0 mmol / mL solution;
[0056] Step 2: Place the Co-Ru / C nanocatalyst prepared in Example 1 in a reactor, add 9 mL of deionized water, seal the reactor and place it in a 303K water bath, and turn on the stirrer to stir;
[0057] Step 3: Use a syringe to draw 1 mL of the sodium borohydride solution prepared in step 1, quickly inject it into the reactor in step 2, start timing at the same time, and record the hydrogen volume at the corresponding time every 30 s.
[0058] Figure 5 The rate diagram for hydrogen production by the hydrolysis of sodium borohydride catalyzed by the Co-Ru / C nanocatalyst prepared in Example 1 of the present invention. It can be seen from the figure that the reaction was completed within...
Embodiment 3
[0060] The Co-Ru / C nanocatalyst prepared according to Example 1 catalyzes sodium borohydride (NaBH 4 ) The specific steps of hydrolysis hydrogen production reaction are as follows:
[0061] In Step 2 of Example 2, seal the reactor and place it in a 303K water bath instead of placing the reactors in a 293K, 313K, and 323K water bath respectively. Other steps are identical with embodiment 2.
[0062] Figure 6 The rate diagram of the Co-Ru / C nanocatalyst prepared in Example 1 of the present invention to catalyze the hydrolysis of sodium borohydride to produce hydrogen under different temperature conditions. It can be seen from the figure that the higher the temperature, the faster the reaction rate. At 323K, the reaction ends within 3 min. According to the hydrogen production rate at four different temperatures, it can be calculated that under the action of Co-Ru / C nanocatalyst, sodium borohydride (NaBH 4 ) The activation energy of hydrolysis hydrogen production reaction is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lattice spacing | aaaaa | aaaaa |
| lattice spacing | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com