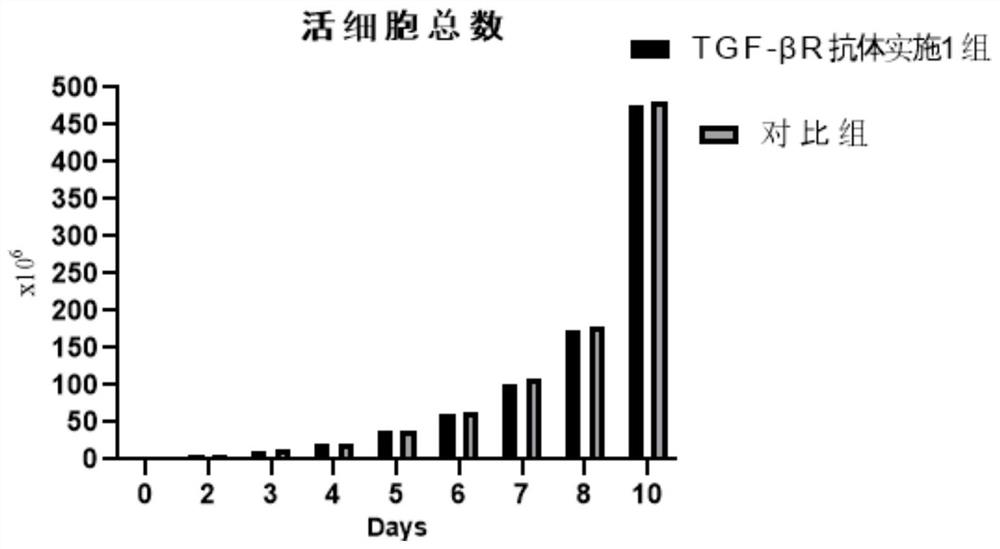
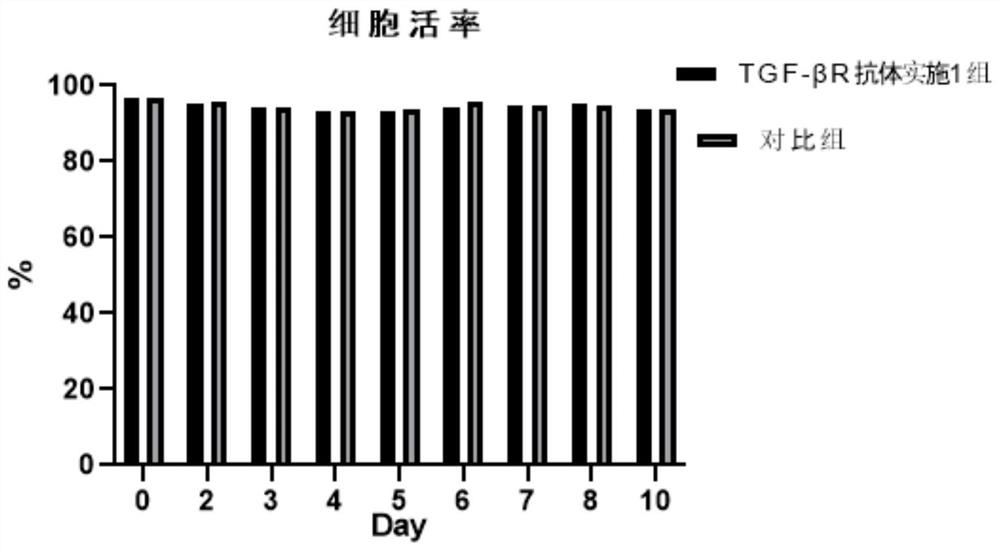
Initiative immune cell activation and amplification system and application thereof
An immune cell and expansion system technology, applied in the field of initial immune cell activation and expansion system, can solve the problem that patients cannot prepare and obtain CAR-T cells in vitro, so as to ensure the preparation quantity and quality, avoid functional decline, avoid Insufficient number of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] 1. Preparation of activated medium
[0079] Preparation method of activation medium: take 15mL PRIME-XV T Cell CDM medium, add CD3 antibody to 100ng / mL, add CD28 antibody to 100ng / mL, add IL-2 to 200IU / mL, add IL-7 to 10ng / mL , add IL-15 to 10 ng / mL, add TGF-βR antibody to 50 ng / mL.
[0080] 2. Isolation of PBMCs
[0081] 2.1 Clinical patient A, with informed consent, collected peripheral blood samples intravenously. Receive the blood sample, wipe the surface with a dust-free cloth soaked in 75% alcohol for disinfection, and put it into a biological safety cabinet.
[0082] 2.2 Take 15mL blood sample and transfer it to a clean and sterile 50mL centrifuge tube.
[0083] 2.3 Add normal saline (NS) for injection to a volume of 30 mL in a 50 mL centrifuge tube, and mix the blood cells evenly.
[0084] 2.4 Transfer 15mL GE Ficoll Lymphocyte Separation Solution to a clean and sterile 50mL centrifuge tube.
[0085] 2.5 After the Ficoll is equilibrated at room temperature,...
Embodiment 2
[0152] 1. Preparation of activated medium
[0153] Preparation method of activation medium: Take 15mL PRIME-XV T Cell CDM medium, add CD3 antibody to 150ng / mL, add CD28 antibody to 75ng / mL, add IL-2 to 250IU / mL, add IL-7 to 15ng / mL , add IL-15 to 15ng / mL, add TGF-βR antibody to 100ng / mL.
[0154] 2. Separation of PBMCs
[0155] 2.1 Clinical patient B, with informed consent, collected peripheral blood samples intravenously. Receive the blood sample, wipe the surface with a dust-free cloth soaked in 75% alcohol for disinfection, and put it into a biological safety cabinet.
[0156] 2.2 Take 15mL blood sample and transfer it to a clean and sterile 50mL centrifuge tube.
[0157] 2.3 Add normal saline (NS) for injection to a volume of 30 mL in a 50 mL centrifuge tube, and mix the blood cells evenly.
[0158] 2.4 Transfer 15mL GE Ficoll Lymphocyte Separation Solution to a clean and sterile 50mL centrifuge tube.
[0159] 2.5 After the Ficoll is equilibrated at room temperature, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com