Method for preparing 2, 2-bis (3-nitro-4-hydroxyphenyl) hexafluoropropane by microchannel reactor
A microchannel reactor, hydroxyphenyl technology, applied in chemical instruments and methods, chemical/physical/physical chemical reactors, preparation of nitro compounds, etc. Exacerbating problems such as rapid reaction, avoiding intermediate separation steps, and reducing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
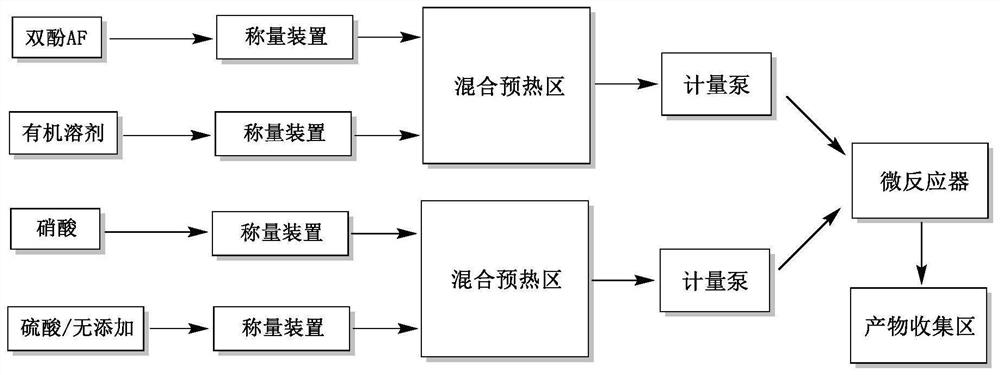
[0030] (1) Refer to figure 1 Determine the connection method, the heat transfer medium of the heat exchanger is heat transfer oil. The diameter of the microchannel reactor is 0.5mm.
[0031] (2) 150g of bisphenol AF and 600g of dichloromethane were mixed and stirred to form a bisphenol AF solution; 86g of nitric acid (concentration 65%) and 172g of sulfuric acid (concentration 98%) were prepared into a mixed acid solution. After the two systems were preheated at 0°C, they were pumped into the microchannel reactor through the metering pump at the flow rate of 25g / min and 8.6g / min respectively. At this time, the molar ratio of bisphenol AF to mixed acid was 1:2, and the reaction temperature was controlled The temperature is 45°C, the residence time is 30s, and the temperature of the reaction product is lowered through the cooling coil to obtain a discharge liquid containing 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane.
[0032] (3) Add 1 L of 5% sodium bicarbonate solutio...
Embodiment 2
[0035] (1) Refer to figure 1 Determine the connection method, the heat transfer medium of the heat exchanger is heat transfer oil. The diameter of the microchannel reactor is 3mm.
[0036] (2) 150g of bisphenol AF and 750g of dichloroethane were mixed and stirred to form a bisphenol AF solution; 108g of nitric acid (concentration 65%) and 324g of sulfuric acid (concentration 95%) were prepared into a mixed acid solution. After the two systems were preheated at 10°C, they were pumped into the microchannel reactor at the flow rate of 15g / min and 7.2g / min respectively through the metering pump. At this time, the molar ratio of bisphenol AF to nitric acid was 1:2.5, and the reaction temperature was controlled The temperature is 30°C, and the residence time is 60s. The temperature of the reaction product is lowered through the cooling coil to obtain a discharge liquid containing 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane.
[0037] (3) Add 1 L of 5% sodium bicarbonate solut...
Embodiment 3
[0039] (1) Refer to figure 1 Determine the connection method, the heat transfer medium of the heat exchanger is heat transfer oil. The diameter of the microchannel reactor is 5mm.
[0040] (2) 150g of bisphenol AF and 900g of chloroform were mixed and stirred to form a bisphenol AF solution; 120g of nitric acid (concentration 70%) and 480g of sulfuric acid (concentration 90%) were prepared into a mixed acid solution. After the two systems were preheated at 20°C, they were pumped into the microchannel reactor at the flow rate of 11.7g / min and 6.7g / min respectively through the metering pump. At this time, the molar ratio of bisphenol AF to nitric acid was 1:3, and the reaction was controlled The temperature was 15° C., and the residence time was 90 s. The temperature of the reaction product was lowered through a cooling coil to obtain a discharge liquid containing 2,2-bis(3-nitro-4-hydroxyphenyl)hexafluoropropane.
[0041](3) Add 1 L of 5% sodium bicarbonate solution to the di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com