Excision enzyme III-driven three-dimensional DNA nano machine and application thereof
A nanomachine and exonuclease technology, applied in the field of biological analysis, can solve the problems of poor sensitivity, complicated operation and high background signal, and achieve the effect of high sensitivity and improved detection sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
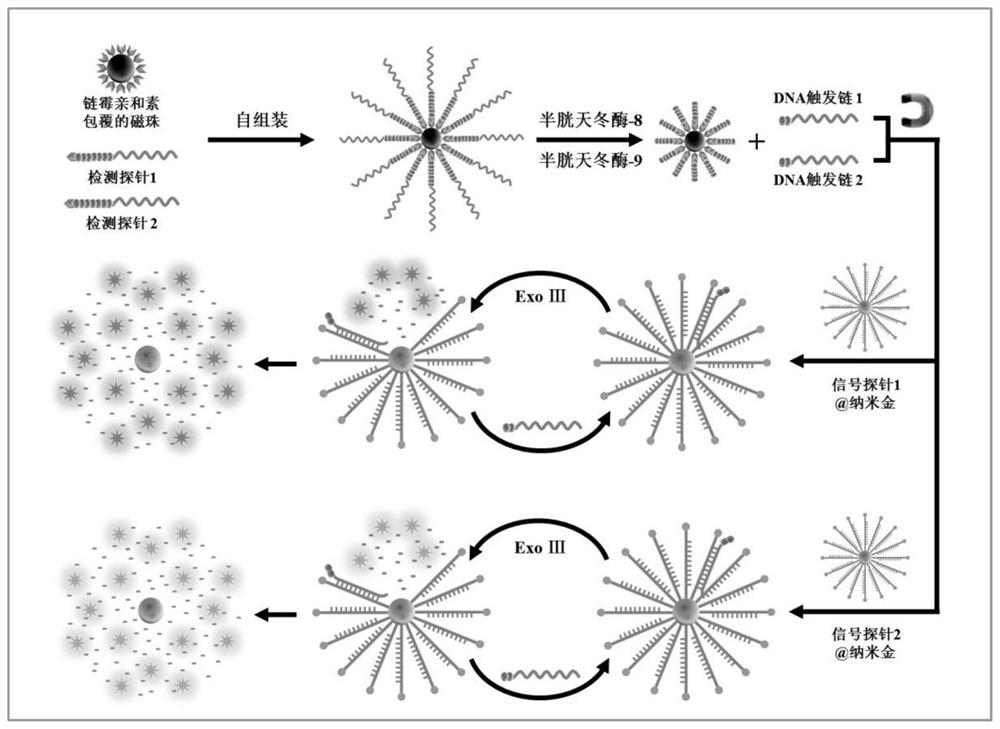
[0061] Example 1 Construction of a three-dimensional DNA nanomachine driven by exonuclease III
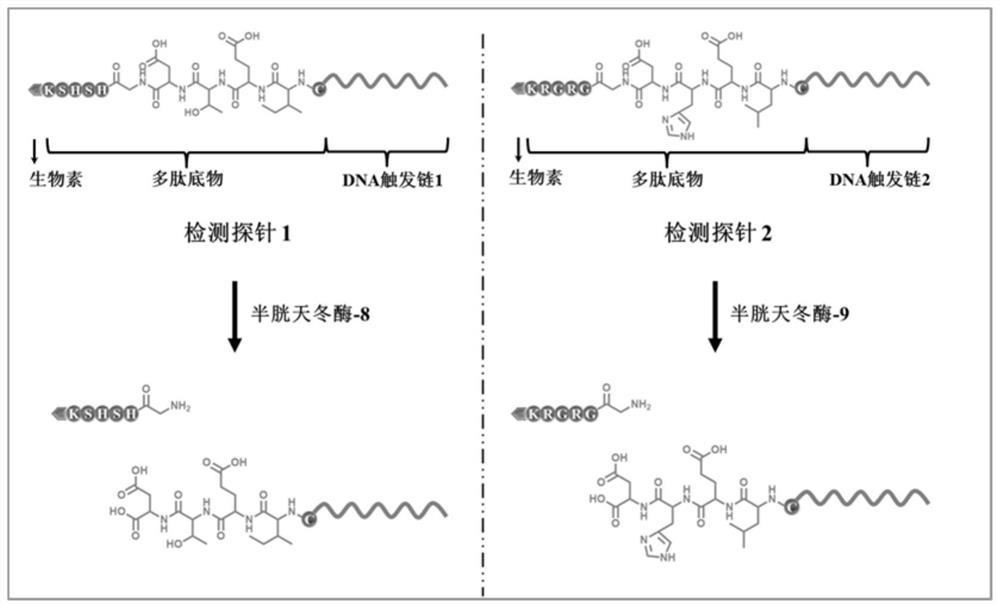
[0062] The detection probe is a polypeptide-DNA detection probe, which includes two types. The polypeptide chain of the detection probe 1 contains the recognition site Ile-Glu-Thr-Asp (IETD) of caspase-8, and its DNA part It can be complementary to signal probe 1; the polypeptide chain of detection probe 2 contains the recognition site Leu-Glu-His-Asp (LEHD) of caspase-9, and its DNA part can be complementary to signal probe 2 .
[0063] The sequence information involved in the exemplary embodiment of the present invention is as shown in Table 1:
[0064] Table 1
[0065]
[0066]
[0067] Note: The recognition site of the target in the detection probe is indicated in bold. The detection probe is a polypeptide-DNA structure, wherein the amino terminal of the polypeptide is modified on the 5' end of the DNA sequence (the structure of carboxyl and amino in the probe struct...
Embodiment 2
[0071] Example 2 Detection of caspase activity and detection of caspase under activity inhibition
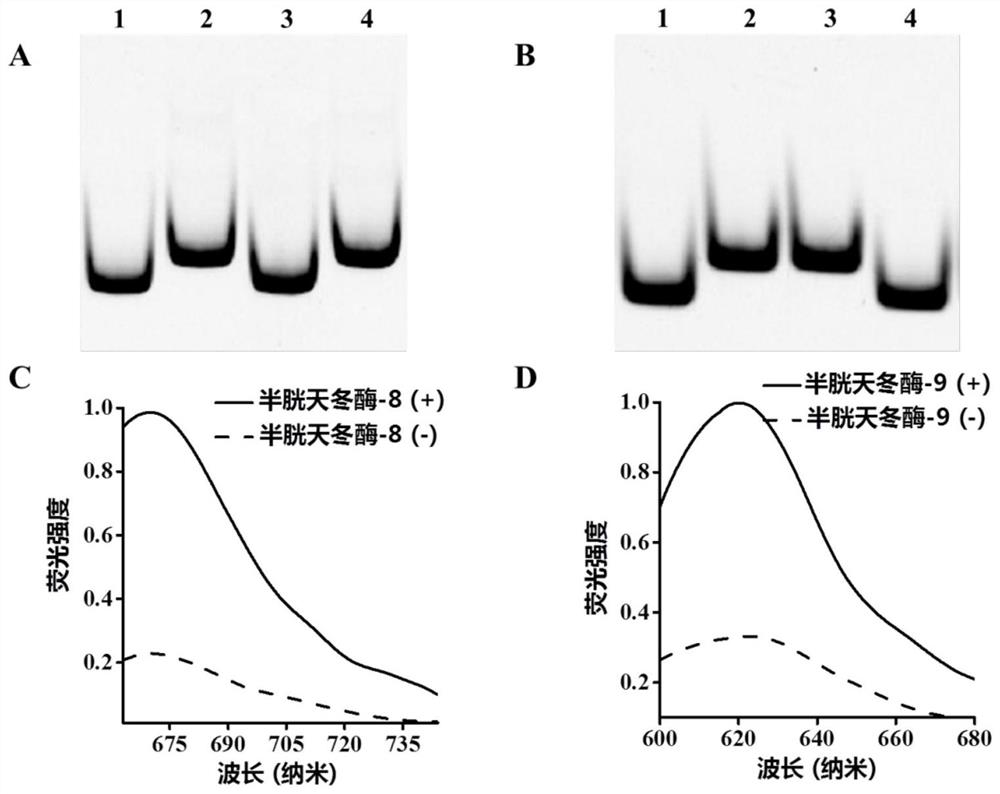
[0072] 1. Detection of caspase activity: the detection of caspase activity consists of two steps, which are the cleavage of the detection probe by caspase and the fluorescence caused by the three-dimensional DNA nanomachine driven by exonuclease III. Cyclic release of molecules. Cleavage of detection probes by caspases was performed in a 20-microliter reaction system containing 11.5 microliters of 1× phosphate buffer solution, 8 microliters of detection probe-magnetic beads and 0.5 units of For caspase-8 or / and caspase-9, incubate at 37°C for 1 hour. After magnetic separation, the supernatant containing the lysed product was taken for the next reaction. In the second step, the reaction system of the three-dimensional DNA walker driven by exonuclease III is 20 μl, which contains 1×Cutsmart buffer (500 mmol per liter of potassium acetate, 200 mmol per liter of tris-acetic acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com