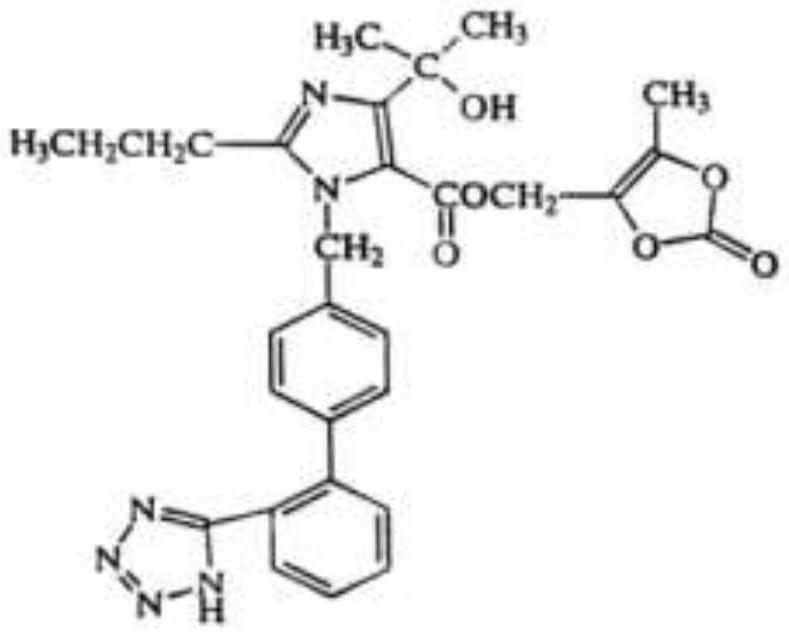
Olmesartan medoxomil dispersible tablet and preparation method thereof
A technology of olmesartan medoxomil and dispersible tablets, which is applied in the field of pharmaceutical preparations, can solve the problems that there are no sustained-release dispersible tablets of olmesartan medoxomil, and achieve the effects of reducing the amount of the main drug, less stimulation, and rapid disintegration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0035] The preparation method of embodiment 1-5 olmesartan medoxomil sustained-release dispersible tablet, the steps are as follows:
[0036] (1) After mixing microcrystalline cellulose, sodium alginate and other qualities, mix them with olmesartan medoxomil and binders that account for a therapeutically effective amount, use water as a solvent, and prepare by centrifugal granulation or extrusion spheronization into olmesartan medoxomil pellets;
[0037] (2) adopt fluidized bed method, in pellet surface coating, the coating liquid that adopts is HPMC coating film; Obtain coating olmesartan medoxomil pellet after coating process;
[0038] (3) The coated olmesartan medoxomil pellets and the remaining olmesartan medoxomil bulk drug and the binder solution in the adjuvant are wet granulated and dried, and lubricants, disintegrants, correction agents are added to the dry pellets. Flavoring agent, glidant, whole grain, compressed tablet.
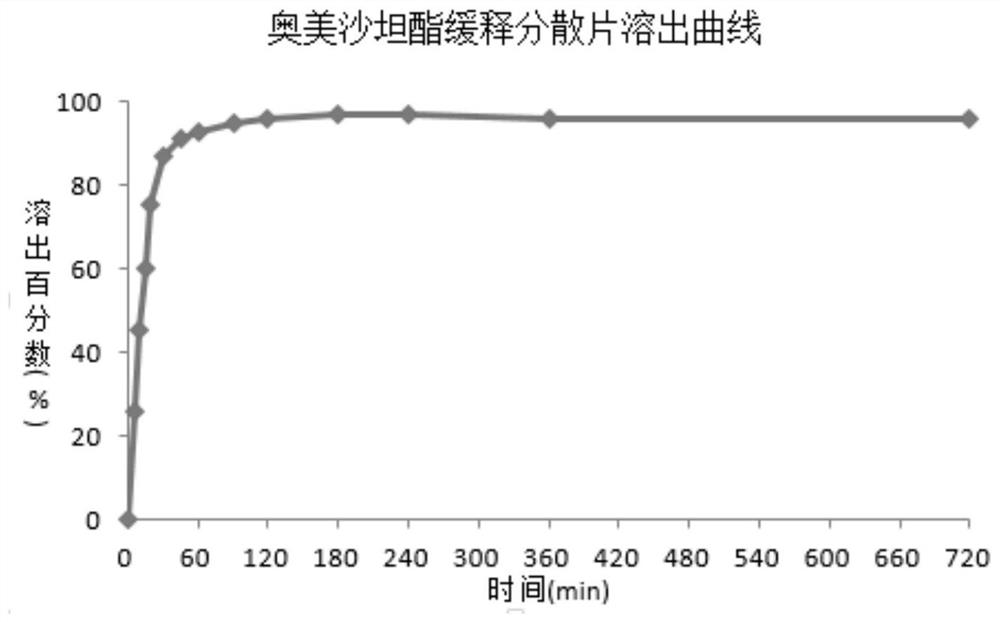
[0039] performance appraisal
[0040] Appe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com