Separation method of hexane isomer
A separation method and isomer technology, which are applied in separation methods, dispersed particle separation, adsorption purification/separation, etc. The effect of large pore volume and adjustable pore structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
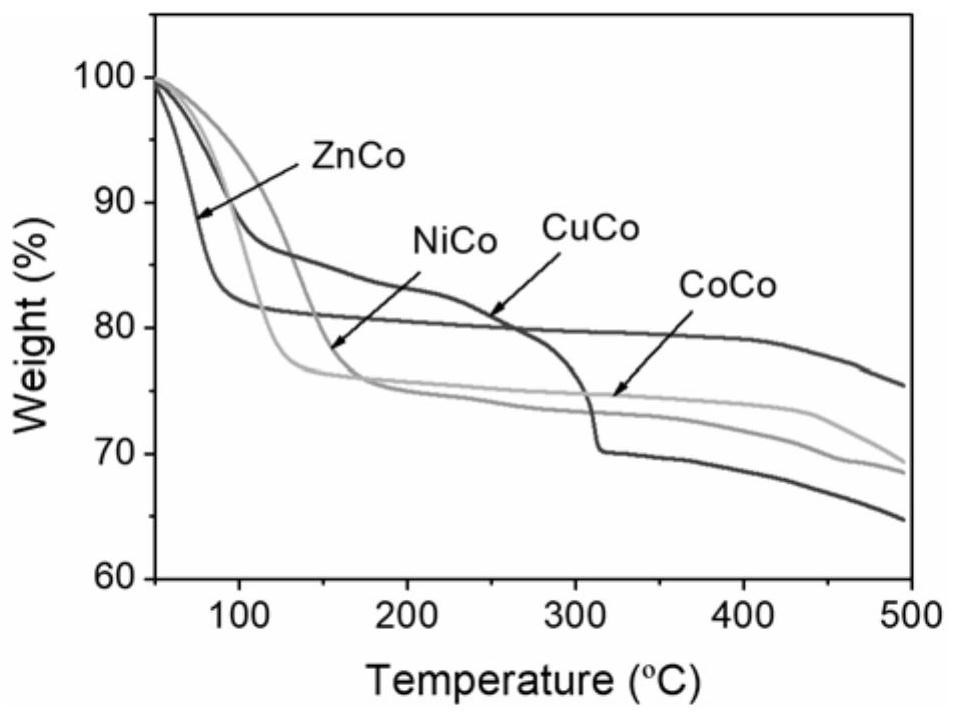
[0050] Mix 10 mmol potassium hexacyanocobaltate with 100 mL deionized water, stir and dissolve to obtain potassium hexacyanocobaltate solution. Mix 18mmol zinc nitrate with 100mL deionized water, stir and dissolve to obtain a zinc nitrate solution. The potassium hexacyanocobaltate solution was added dropwise to the vigorously stirred zinc nitrate solution for reaction. After the dropwise addition, it was aged for 24 hours, and washed with deionized water several times to obtain a purified metal organic framework material. The purified metal organic framework material was vacuum degassed at 150 °C for 24 hours to obtain the metal organic framework material Zn 3 [Co(CN) 6 ] 2 . After testing, Zn 3 [Co(CN) 6 ] 2 It is a hexagonal crystal with a large window size of Specific surface area 863m 2 / g. Gas adsorption followed.
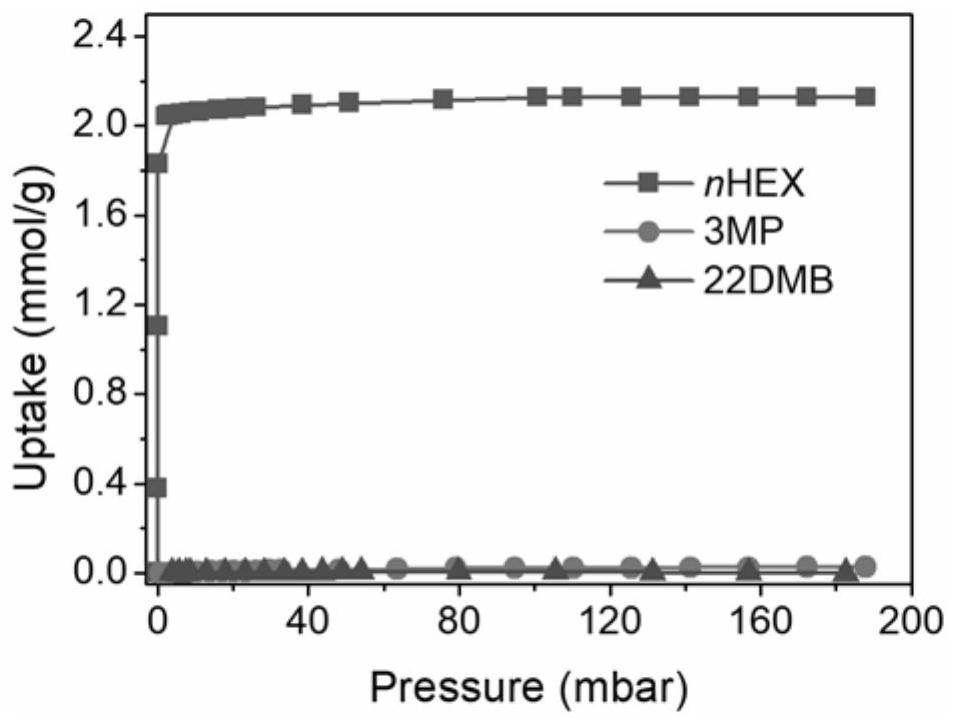
[0051] In order to test the practical effect of the above-synthesized MOFs on the separation of hexane isomers, the above-synthesized MOFs Zn 3 [...
Embodiment 2
[0055] Mix 10 mmol potassium hexacyanocobaltate with 100 mL deionized water, stir and dissolve to obtain potassium hexacyanocobaltate solution. Mix 18 mmol of nickel nitrate with 100 mL of deionized water, stir and dissolve to obtain a nickel nitrate solution. The potassium hexacyanocobaltate solution was added dropwise to the vigorously stirred nickel nitrate solution for reaction. After the dropwise addition, it was aged for 24 hours, and washed with deionized water several times to obtain a purified metal organic framework material. The purified MOFs were degassed under vacuum at 150 °C for 24 hours to obtain the desolvated MOFs Ni 3 [Co(CN) 6 ] 2 , after testing, Ni 3 [Co(CN) 6 ] 2 It is a cubic crystal form, and the size of the macropore window is Gas adsorption followed.
[0056] In order to test the practical effect of the above-synthesized metal-organic framework material on the separation of hexane isomers, the above-synthesized metal-organic framework mate...
Embodiment 3
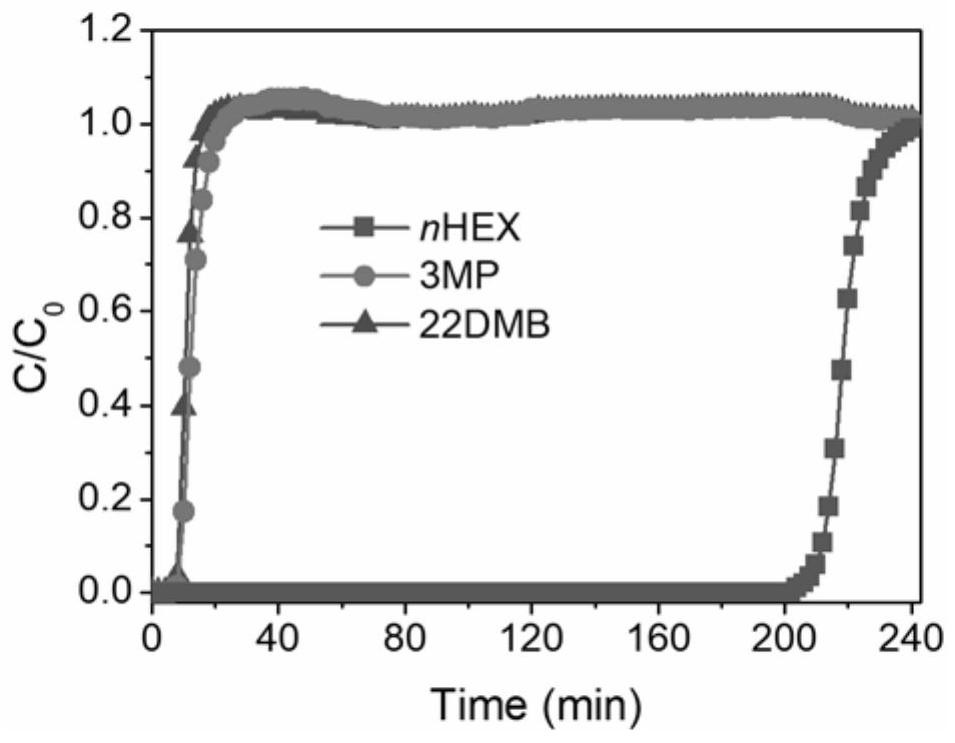
[0066] The material prepared in Example 1 is subjected to a three-component gas mixture at normal temperature: n-hexane, 3-methylpentane, 2,2-dimethylbutane, nitrogen (nitrogen is an inert component) gas mixture circulation Regeneration penetration experiments. The result obtained is as Figure 12 It is shown that the material has excellent recycling performance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com