Method for producing light aromatic hydrocarbon
A technology for light aromatic hydrocarbons and products, applied in the field of light aromatic hydrocarbon production, can solve the problems of low yield of light aromatic hydrocarbons, etc., and achieve the effects of high selectivity, content reduction, and economical improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 produces the method for light aromatics
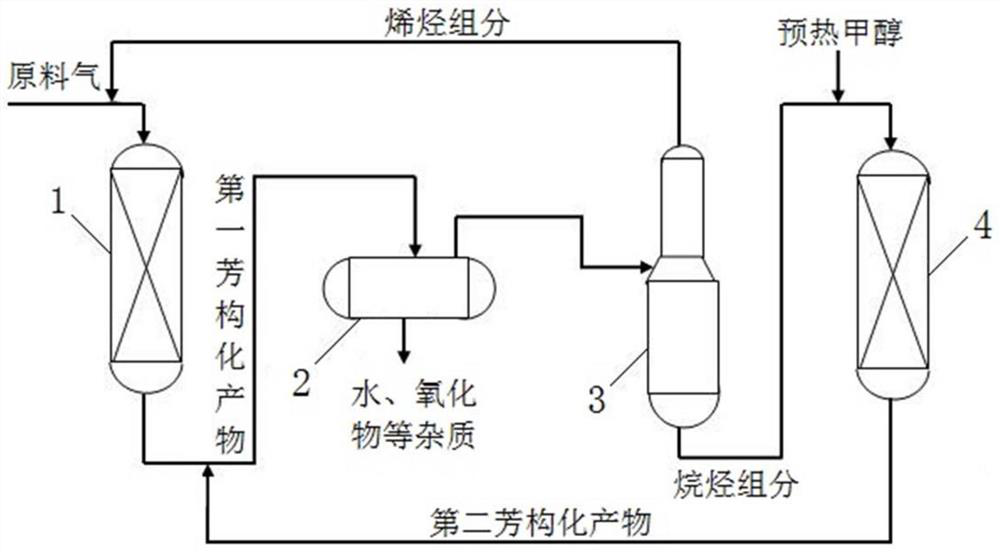
[0048] figure 1 It is a schematic diagram of the technological process for increasing the production of light aromatics in this embodiment, such as figure 1 As shown, the method for producing light aromatics in the present embodiment comprises the steps:
[0049] 1) In the presence of the first catalyst, perform a first aromatization reaction on the material to be reacted including low-carbon oxygen-containing organic compounds to obtain a first aromatization product;
[0050] Specifically, the materials to be reacted and the dilution gas are mixed according to a certain mass ratio (to form raw material gas), and after being properly preheated, they are passed into the first reactor 1 equipped with the first catalyst, and the materials to be reacted are contacted with the first catalyst, Under certain conditions of temperature, pressure (inlet pressure of the reactor), and mass space velocity (including the mass s...
Embodiment 2
[0060] The preparation of embodiment 2 catalyst
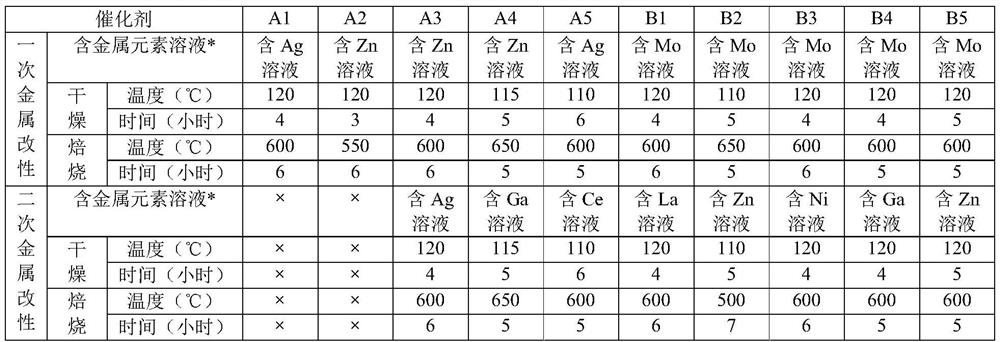
[0061] Prepare the first catalyst A1-A5, the second catalyst B1-B5 according to the following process:
[0062] 1) A1-A2 preparation process:
[0063] Primary metal modification: ZSM-5 molecular sieves are impregnated with equal volumes in a solution containing the first metal element, followed by drying and calcining to obtain the corresponding first catalyst.
[0064] 2) A3-A4 preparation process:
[0065] (1) Primary metal modification: the ZSM-5 molecular sieve is impregnated with an equal volume of a solution containing the first metal element, followed by drying and roasting to obtain a metal-modified ZSM-5 molecular sieve;
[0066] (2) Secondary metal modification: using a solution containing the first metal element to impregnate the metal-modified ZSM-5 molecular sieve with an equal volume, followed by drying and roasting to obtain the corresponding first catalyst.
[0067] 3) B1-B5 preparation process:
[0068] (1)...
Embodiment 3- Embodiment 7
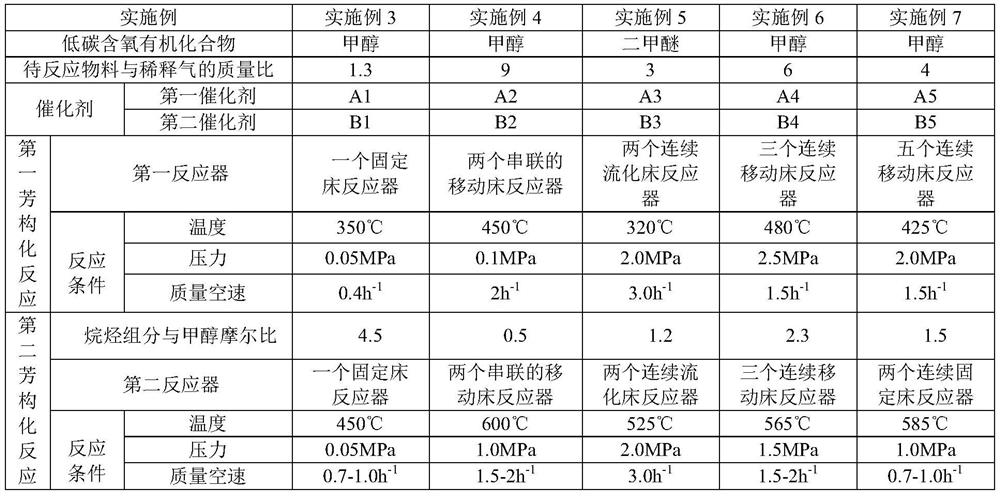
[0077] Embodiment 3-Example 7 respectively use the first catalysts A1-A5 / second catalysts B1-B5 of Example 2, and produce aromatics according to the method of Example 1.
[0078] Specifically, in Example 3-Example 7, the low-carbon oxygen-containing organic compound used, the mass ratio of the material to be reacted and the diluent gas, the first reactor, and the temperature in the first aromatization reaction condition (ie step 1) , pressure, mass space velocity), the second reactor, the mol ratio of alkane component and preheated methanol, the reaction parameters such as temperature, pressure, mass space velocity) in the second aromatization reaction conditions (ie step 4) are all as Shown in Table 3, the product composition of the final reaction product of embodiment 3-embodiment 8 is shown in table 4.
[0079]The reaction parameter of table 3 embodiment 3-embodiment 7
[0080]
[0081] Table 4 embodiment 3-embodiment 7 final reaction product composition
[0082]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com