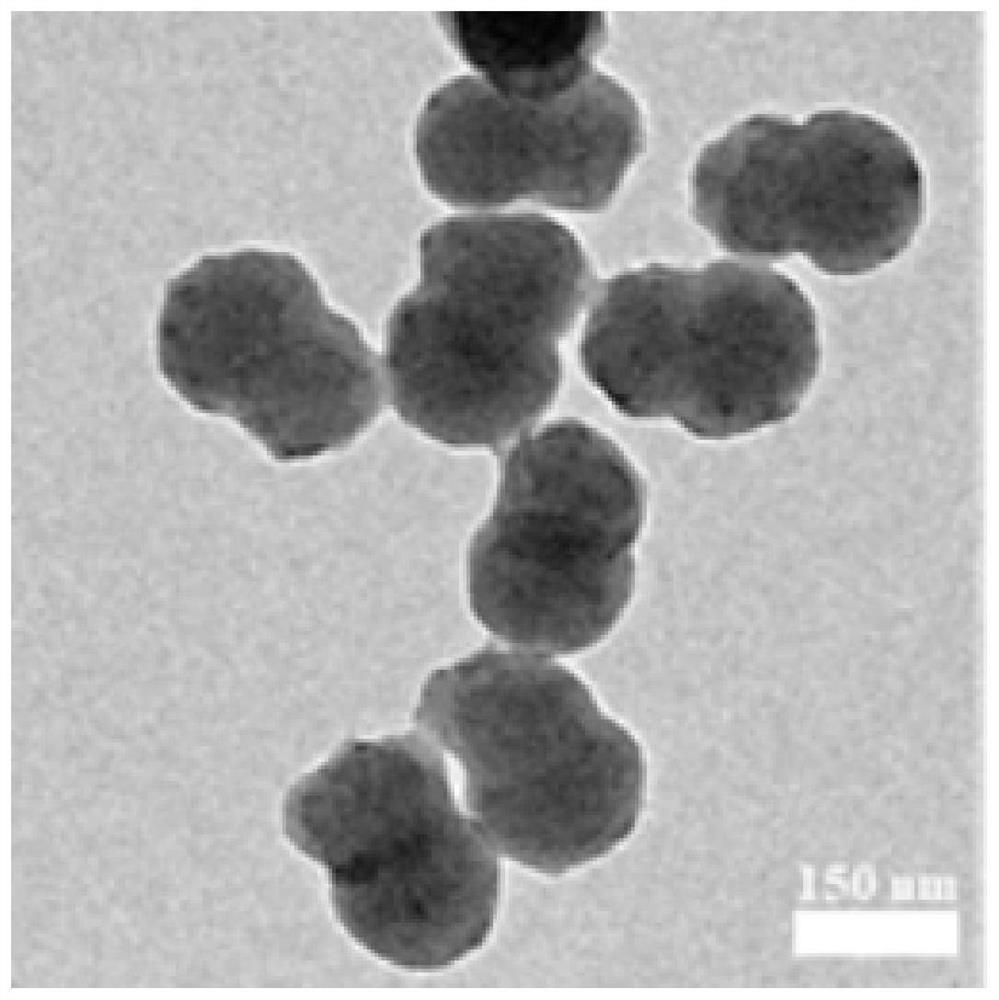
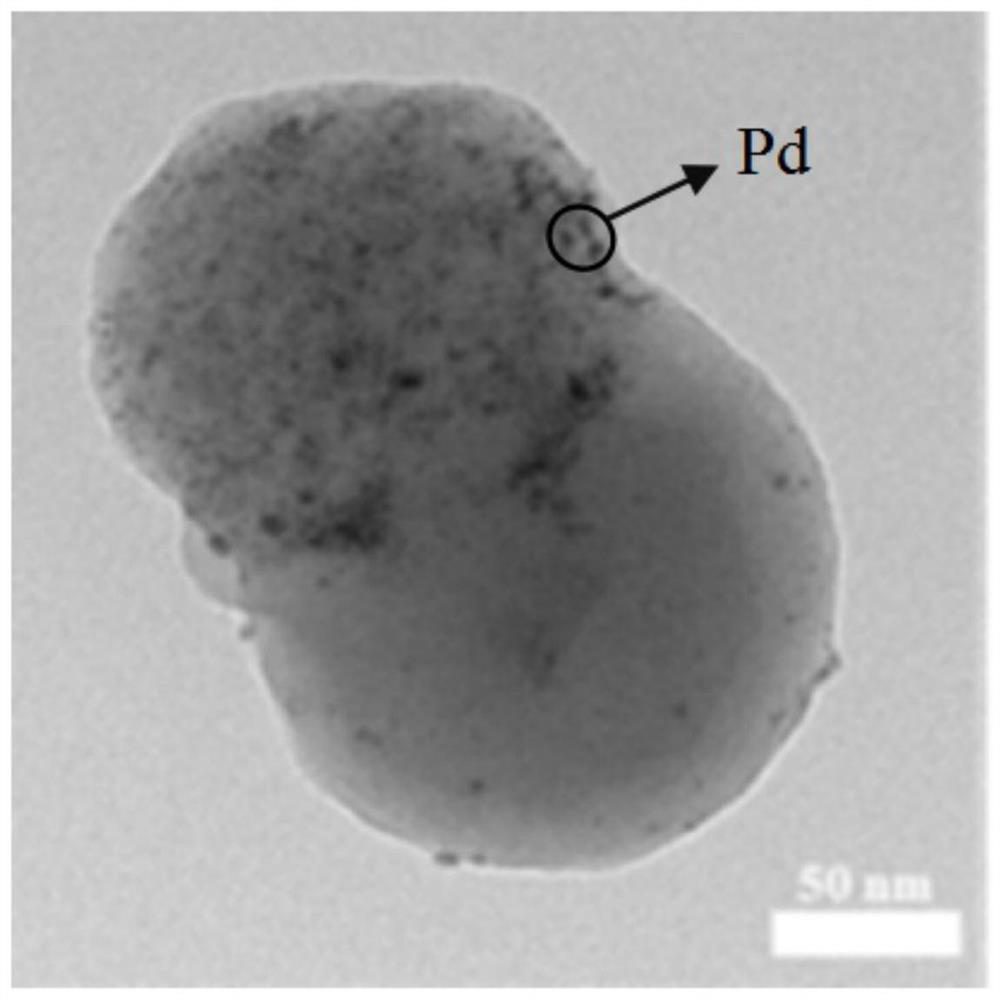
Janus structure polymer-based nano-metal catalyst as well as preparation method and application thereof
A technology of structural polymers and nano-metals, which is applied in the direction of catalyst activation/preparation, preparation of organic compounds, organic compound/hydride/coordination complex catalysts, etc. It can solve the problems of chemical composition partitioning and microstructure precise control technology. Improvement and other issues, to achieve good emulsification effect, shorten the preparation time, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Weigh 0.13g sodium lauryl sulfate, 125mL H 2 O is dissolved in the reactor under room temperature with mechanical stirring, while adding 10.42g styrene and 0.33g divinylbenzene, and continuously feeding N 2 After 30 minutes, the temperature of the reaction system was raised to 80°C, and the mixed solution of 0.19g potassium persulfate and 19mL water was slowly added dropwise, and the polymerization was carried out at constant temperature for 10 hours. After the reaction was completed, the reaction solution was cooled to room temperature, and 60 mL of H 2 O, 6.04g p-chloromethyl styrene, 0.12g divinylbenzene, continue to swell for 4h and then raise the temperature to 60°C, and add dropwise 0.24g potassium persulfate, 0.18g NaHSO 3 with 15mL H 2 The mixed solution of O, continuous reaction 5h obtains seed emulsion;
[0044] Weigh 10g of the above seed emulsion, 10g of H 2 O and 0.33g sodium lauryl sulfate in a reactor, mechanically stirred until sodium lauryl sulfate ...
Embodiment 2
[0047] Weigh 0.13g sodium lauryl sulfate, 125mL H 2 O is dissolved in the reactor under room temperature with mechanical stirring, while adding 10.42g styrene and 0.33g divinylbenzene, and continuously feeding N 2 After 30 minutes, the temperature of the reaction system was raised to 80°C, and the mixed solution of 0.19g potassium persulfate and 19mL water was slowly added dropwise, and the polymerization was carried out at constant temperature for 10 hours. After the reaction was completed, the reaction solution was cooled to room temperature, and 60 mL of H 2 O, 6.04g p-chloromethyl styrene, 0.12g divinylbenzene, continue to swell for 4h and then raise the temperature to 60°C, and add dropwise 0.24g potassium persulfate, 0.18g NaHSO 3 with 15mL H 2 The mixed solution of O, continuous reaction 5h obtains seed emulsion;
[0048] Take by weighing 20g above-mentioned seed emulsion, 20g H 2 O and 0.66g sodium lauryl sulfate in a reactor, mechanically stirred until the sodium ...
Embodiment 3
[0050] Weigh 0.22g sodium lauryl sulfate, 125mL H 2 O was dissolved in the reactor under mechanical stirring at room temperature, while adding 20.83g styrene, 0.33g divinylbenzene, and continuously feeding N 2 After 30 minutes, the temperature of the reaction system was raised to 80°C, and the mixed solution of 0.4g potassium persulfate and 30mL water was slowly added dropwise, and the polymerization was carried out at constant temperature for 12h. After the reaction was completed, the reaction solution was cooled to room temperature, and 100 mL of H 2 O, 12.2g p-chloromethyl styrene, 0.25g divinylbenzene, continue to swell for 6h and then raise the temperature to 60°C, and add dropwise 0.24g potassium persulfate, 0.18g NaHSO 3 with 15mLH 2 The mixed solution of O is continuously reacted for 8h to obtain the seed emulsion;
[0051] Take by weighing 20g above-mentioned seed emulsion, 40g H 2 O and 1.32g sodium lauryl sulfate in the reactor, mechanical stirring until sodium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com