Human NK cell culture system and preparation method thereof
A culture system, NK cell technology, applied in cell culture active agents, cell culture supports/coatings, biochemical equipment and methods, etc., can solve the risk of increasing clinical application, low amplification efficiency and purity, and poor repeatability To achieve the effect of promoting progenitor cell proliferation, improving cell purity, and reducing the probability of contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 In vitro expansion of human umbilical cord blood NK cells
[0055] 1. Coated culture flask: 24-48 hours before inoculation, mix human hepatitis B immunoglobulin (50μg / mL) and human serum albumin (200μg / mL) according to the ratio of 1:1, take 10mL and add it to the 75cm 2 In a culture bottle, mix well, let it stand at room temperature for 1 hour, and then put it in a refrigerator at 4°C for later use.
[0056] Before use, discard the supernatant in the coated culture flask and wash it again with PBS.
[0057] 2. Gradient centrifugation of umbilical cord blood and separation of umbilical cord blood mononuclear cells and plasma:
[0058] (1) Blood collection: Umbilical cord blood was taken from the umbilical cord of full-term healthy newborns, anticoagulated with sodium citrate, and separated within 24 hours after collection.
[0059] (2) Separation: Cord blood was diluted according to the ratio of cord blood: PBS = 1:1, slowly added to an equal volume of human...
Embodiment 2
[0073] Example 2 In vitro expansion of human umbilical cord blood NK cells
[0074] 1. Coated culture flask: 24-48 hours before inoculation, mix human hepatitis B immunoglobulin (100μg / mL) and human serum albumin (500μg / mL) according to the ratio of 1:1, take 10mL and add 75cm 2 In a culture bottle, mix well, let it stand at room temperature for 1 hour, and then put it in a refrigerator at 4°C for later use.
[0075] Before use, discard the supernatant in the coated culture flask and wash it again with PBS.
[0076] 2. Gradient centrifugation of umbilical cord blood and separation of umbilical cord blood mononuclear cells and plasma:
[0077] (1) Blood collection: Umbilical cord blood was taken from the umbilical cord of full-term healthy newborns, anticoagulated with sodium citrate, and separated within 24 hours after collection.
[0078] (2) Separation: Cord blood was diluted according to the ratio of cord blood: PBS = 1:1, slowly added to an equal volume of human lymphocy...
experiment example 1
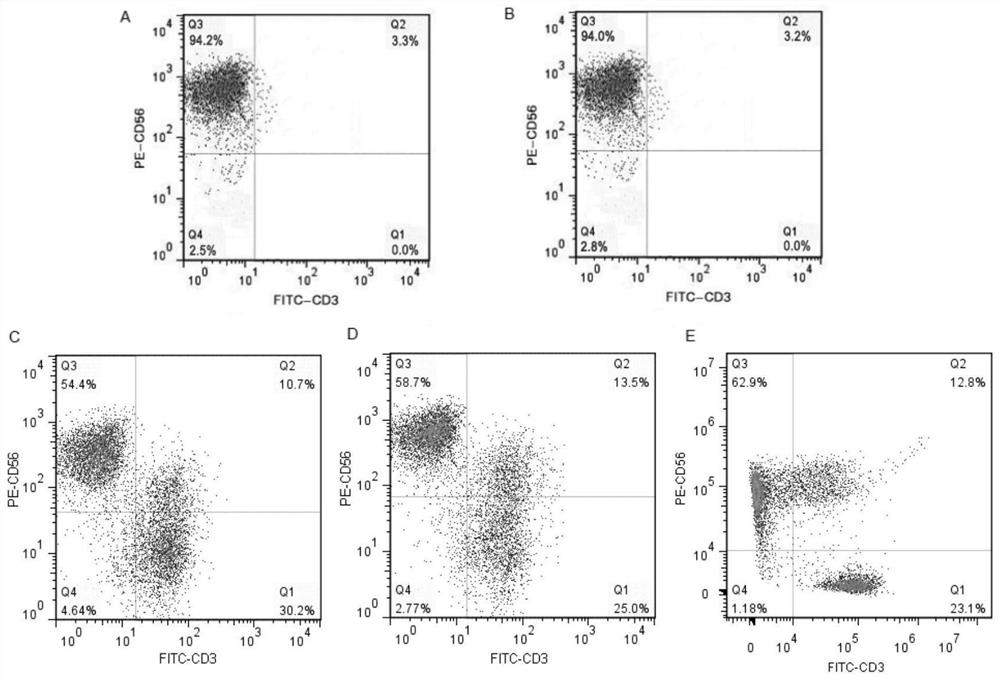
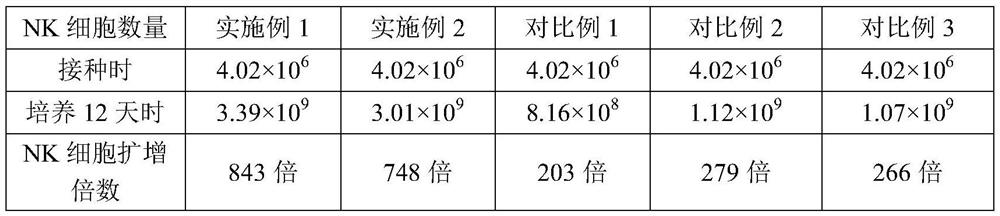
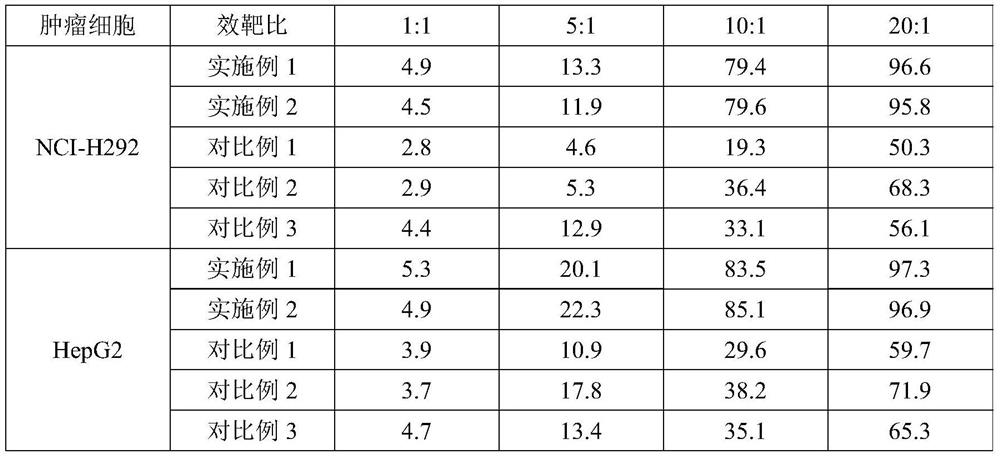
[0098] Experimental example 1 cell count
[0099] On the 0th and 12th day of cell culture, samples were taken and counted respectively, and the test results are shown in Table 1 below.
[0100] Table 1 Total cell count results
[0101] cell number Day 0(unit) Day 12 (month) Example 1 2×10 7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com