Method for photocatalytic synthesis of quinazolinone
A quinazolinone, photocatalytic technology, applied in chemical instruments and methods, catalytic reactions, physical/chemical process catalysts, etc., can solve the problems of high price and low reserves, and achieve short reaction time, high academic value, and reaction mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: 2-phenyl-4-quinazolinone
[0031] 2-Phenyl-4-quinazolinone
[0032]
[0033] Anthranilamide (27.2 mg, 0.2 mmol), benzaldehyde (21.2 mg, 0.2 mmol), fluorescein (1.3 mg, 2 mol%), p-toluenesulfonic acid (3.4 mg, 10 mol%), and acetonitrile (2 mL) were sequentially added to a 15 mL reaction tube. The reaction mixture was reacted under the irradiation of 10 w blue light for 2 h. After the reaction, the solvent was removed by rotary evaporation, and then the pure The target compound was a white solid (39.1 mg) with a melting point of 237-238°C.
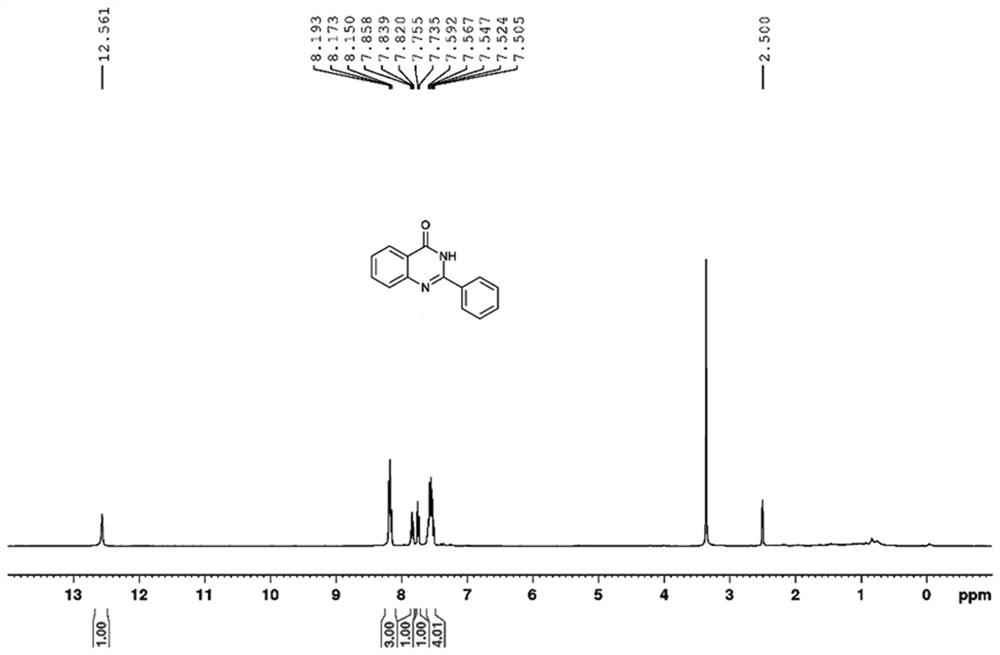
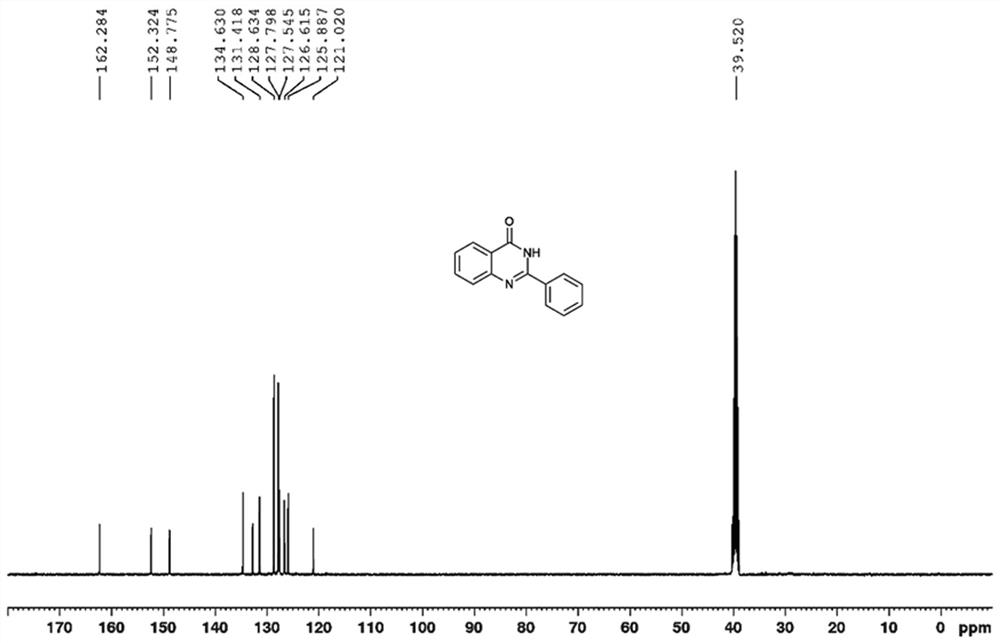
[0034] The NMR spectrum of the product is as figure 1 and 2 As shown, the details are as follows:
[0035] 1 H NMR (400 MHz, [D 6 ]DMSO) δ 12.56 (br s, 1H), 8.19-8.15 (m, 3H), 7.84(t, J = 7.5 Hz, 1H), 7.76 (d, J = 8.1 Hz, 1H), 7.59-7.51 (m, 4H); 13 C NMR (100MHz, [D 6 ]DMSO) δ 162.3, 152.3, 148.8, 134.6, 131.4, 128.6, 127.8, 127.5, 126.6, 125.9, 121.0, 39.5.
Embodiment 2
[0036] Example 2: 2-phenyl-4-quinazolinone
[0037] 2-Phenyl-4-quinazolinone
[0038]
[0039] Anthranilamide (27.2 mg, 0.2 mmol), benzaldehyde (21.2 mg, 0.2 mmol), fluorescein (1.3 mg, 2 mol%), p-toluenesulfonic acid (10.2 mg, 30 mol%), and acetonitrile (2 mL) were sequentially added to a 15 mL reaction tube. The reaction mixture was reacted under the irradiation of 10 w blue light for 2 h. After the reaction, the solvent was removed by rotary evaporation, and then the pure The target compound was a white solid (39.1 mg) with a melting point of 237-238°C.
Embodiment 3
[0040] Example 3: 2-phenyl-4-quinazolinone
[0041] 2-Phenyl-4-quinazolinone
[0042]
[0043] Anthranilamide (27.2 mg, 0.2 mmol), benzaldehyde (21.2 mg, 0.2 mmol), fluorescein (0.7 mg, 1 mol%), p-toluenesulfonic acid (3.4 mg, 10 mol%), and acetonitrile (2 mL) were sequentially added to a 15 mL reaction tube. The reaction mixture was reacted under the irradiation of 10 w blue light for 2 h. After the reaction, the solvent was removed by rotary evaporation, and then the pure The target compound was a white solid (35.3 mg) with a melting point of 237-238°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com