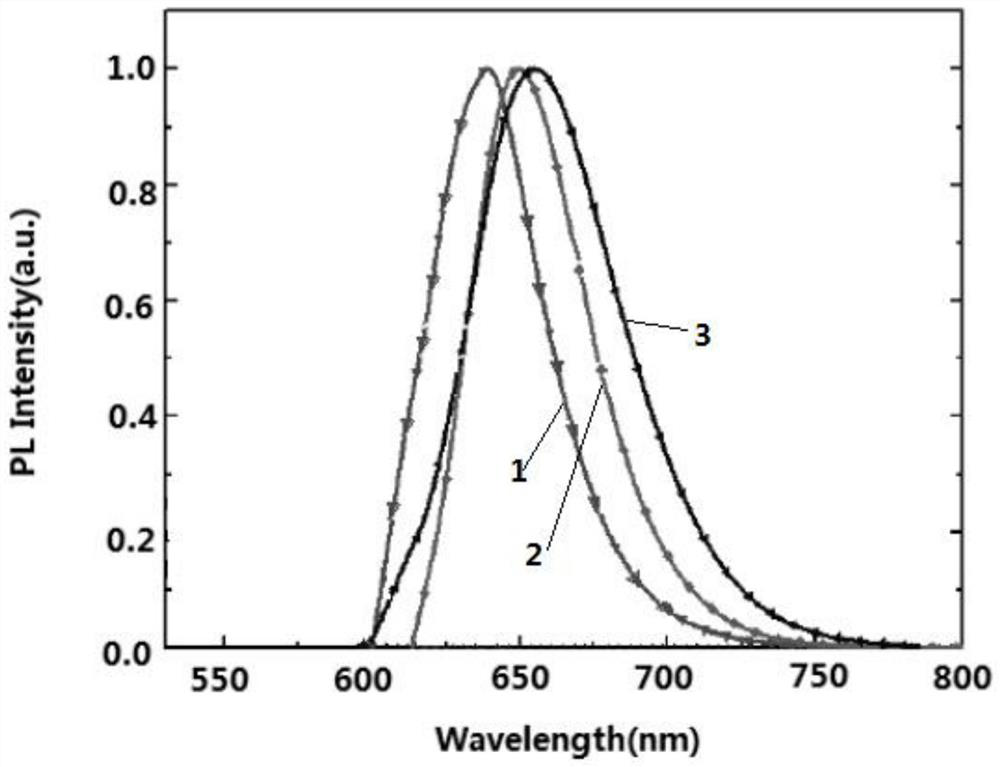
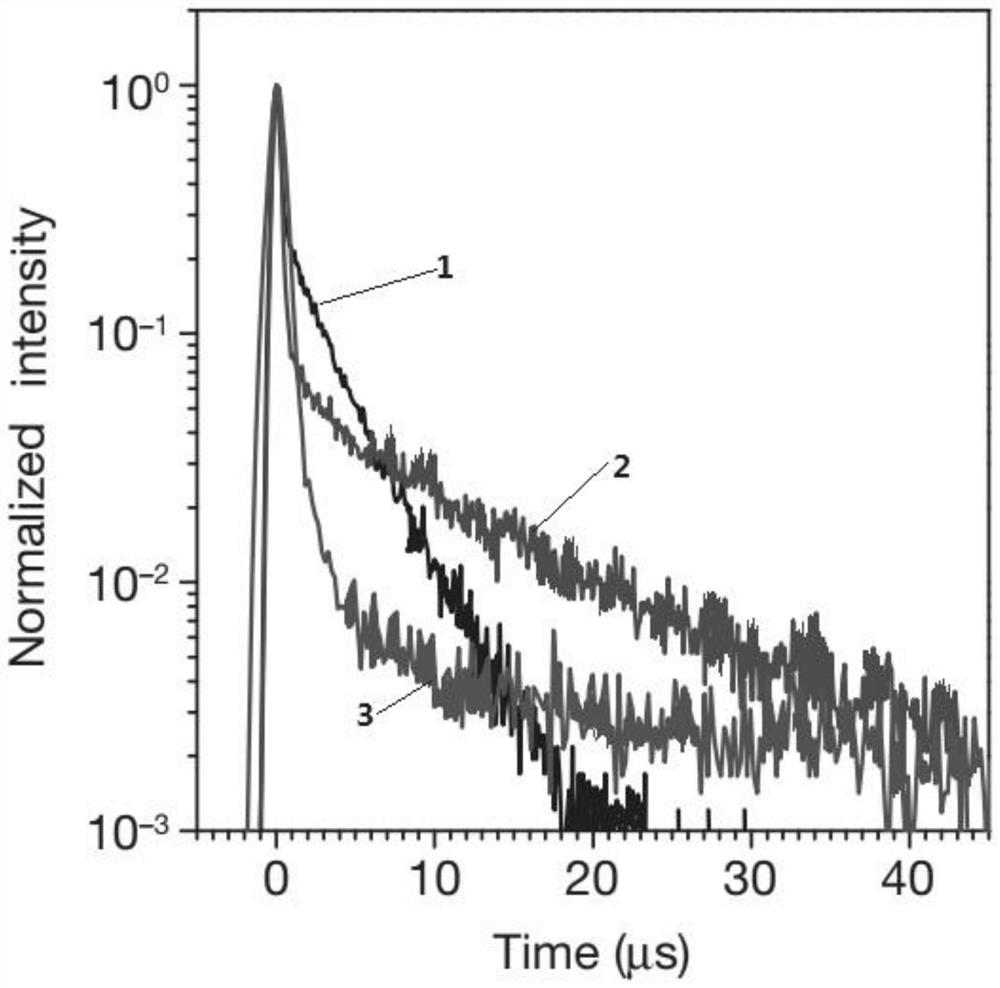
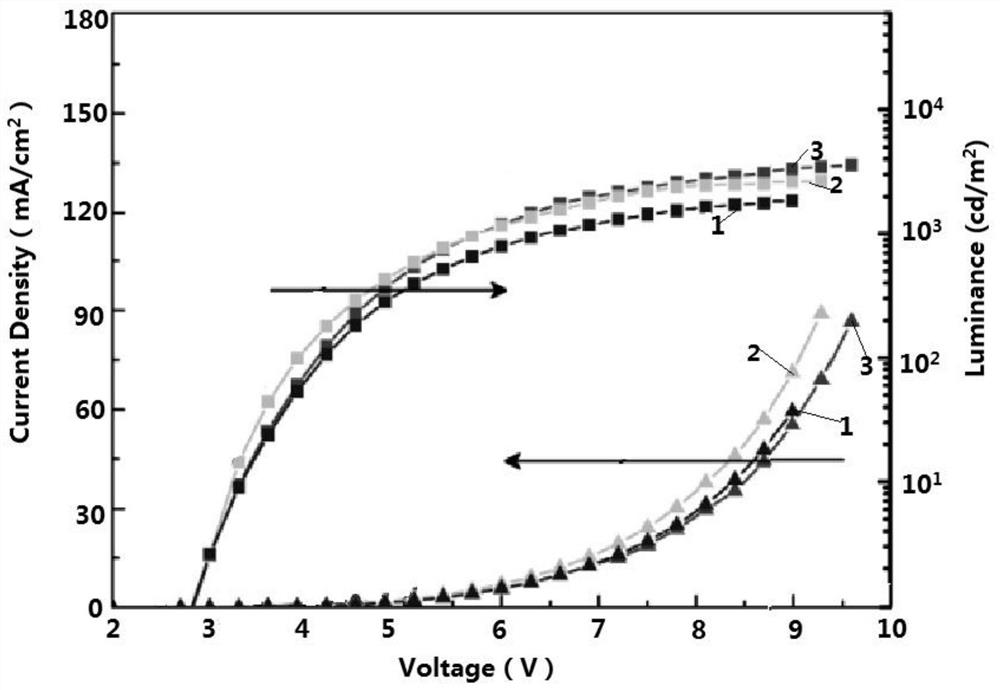
Thermally activated delayed fluorescent material and preparation method thereof
A technology of thermally activated delayed and fluorescent materials, applied in the fields of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of difficult synthesis of red TADF materials and low device efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Pyrene (2.02g, 10mmol) was dissolved in 50ml of hydrogen peroxide, and hydrogen bromide (1.21g, 15mmol), a mixed solution of ether and methanol (volume ratio=1:2) was added to the above solution, at 0°C After stirring for 8h, the obtained product was recrystallized in ethanol to obtain the product 1-bromopyrene (2.38g, yield 85%), and its chemical reaction equation was:
[0019]
Embodiment 2
[0021] 1-bromopyrene (2.81g, 10mmol), 2-(dimethyl-13-oxyoxyalkyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborin (3.46gg, 20mmol), 100ml of anhydrous tetrahydrofuran and butyllithium (40mmol) were added to a 250ml three-necked flask, and after argon was introduced for 10 minutes, the temperature was lowered to -78°C for 28 hours of reaction. After the reaction was completed, extraction and purification were performed to obtain The product 4,4,5,5-tetramethyl-2-(pyran-1-yl)-1,3,2-dioxaborolane (2.33g, yield 71%), its chemical reaction equation is :
[0022]
Embodiment 3
[0024] Anthracene-9,10-dione (1.36g, 10mmol), liquid bromine (4.795g, 30mmol), and 50ml of acetic acid were added to a 100mL three-necked round-bottomed flask, heated to reflux and stirred in an argon atmosphere, and reacted at 80°C 10h. Stop the reaction and cool to room temperature, wash the reaction solution with water 2 to 3 times, concentrate the reaction solution and purify it through a chromatographic column, silica gel 200 to 300 mesh, and the eluent is petroleum ether / ethyl acetate (1:4), to obtain the product 2,7-dibromoanthracene-9,10-dione (2.85g, yield 78%), its chemical reaction equation is:
[0025]
PUM
| Property | Measurement | Unit |
|---|---|---|
| External quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com