Preparation method of benzoyl-containing acrylic acid podophyllotoxin ester derivative and application of benzoyl-containing acrylic acid podophyllotoxin ester derivative in tumor suppression
A technology of benzoylacrylic acid and podophyllotoxin, which is applied in the direction of antineoplastic drugs, organic chemical methods, drug combinations, etc., can solve the problems of strong toxicity, application limitations, and lack of tumor tissue selectivity, and achieve low toxicity and strong side effects. Inhibitory activity, effect of significant tumor cell inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0025] Example 1: Preparation of acetate derivatives of formula I benzoyl acrylic acid
[0026] The catalyst 0.02 mol of anhydrous aluminum chloride and 0.01 mol of syartnutic anhydride was dissolved in 40 mL of flask, stirred at room temperature for 10 min, allowing anhydrous aluminum chloride and tobi-oxyleneic anhydride to fully dissolve, and then 0.01 mol The xylene was slowly added dropwise to the solution system, and the color of the solution gradually became a yellowish brown. The above reaction system was stirred under room temperature for 12 h, and the TCL codes were detected to detect the reaction progress. After the reaction was completed, the concentration of 20% dilute hydrochloric acid was added dropwise to the reaction system to the solution pH = 6 to 7 (neutralized acidity), followed by extraction separation using 100 ml of liquid solution (addition of saturated saline to prevent emulsification) . Extraction three times. The lower dichloromethane solvent layer was ...
example 2
[0042] Example 2: Application of Toxin Toxide Derivatives Toxide Derivatives
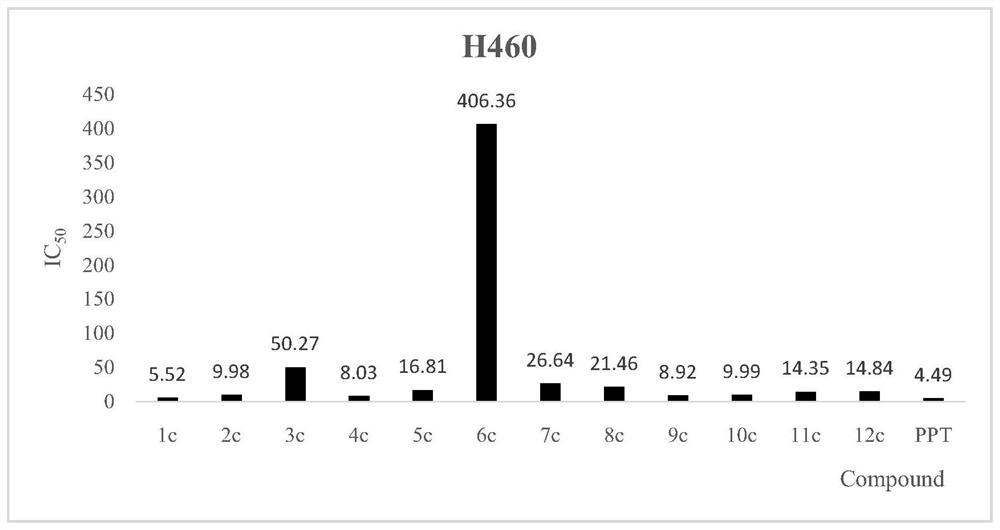
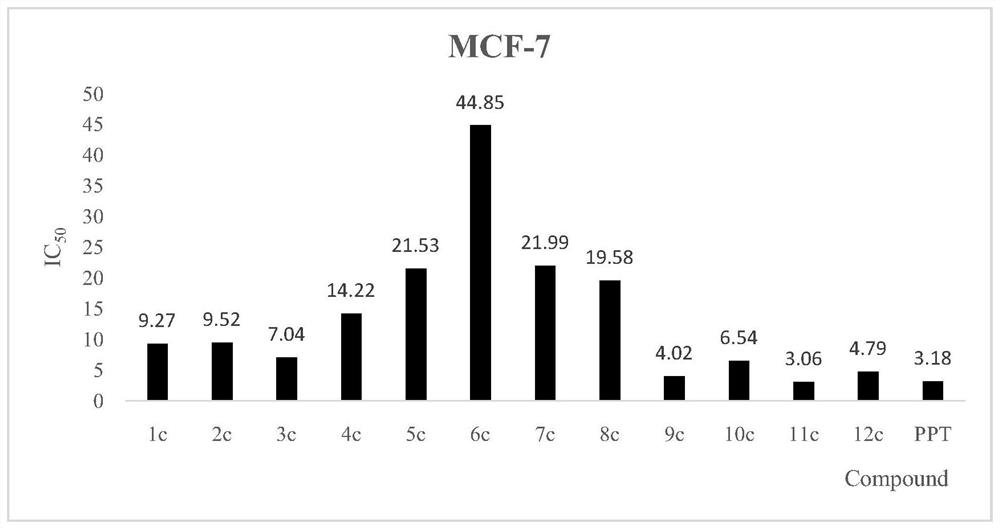
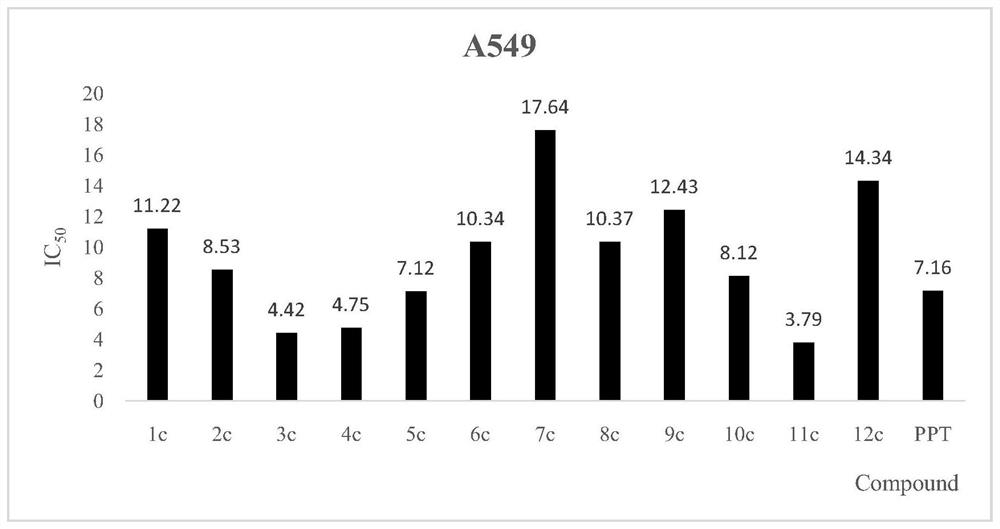
[0043] Taking MCF-7, H460, HGC-27, A549 and 293T human normal renal epithelial cell lines as the detection strain, MTT colorimetric method is a detection method, by tumors of ghostane-derived derivatives in formula I benzoyl acrylic acid Cell inhibitory activity studies have found that the novel structure derivatives have significant inhibitory activity in vitro tumor cells. See the results figure 1 , 2 3, 4.
example 3
[0044] Example 3: Compound 12C significantly induces HGC-27 apoptosis
[0045] The compound 12c was used to HGC-27 cells at different concentrations (0, 0.2, 0.6, 1.2 μm), treated 24 h, collected cells, centrifugation, washed twice with PBS; bindingBuffer in PI / FITC dye kits. The cells were added to 5 μl of FITC and 10 μl Pi, respectively, and the anti-lighting was stained with 4 minutes, and the cell apoptosis was detected with flow cytometry. See the results Image 6 . The novel derivatives of this type of structure can significantly promote the apoptosis of HGC-27 in human gastric cancer cells.
[0046] The benzoyl acrylic acid-ghostophenin ester derived in the present invention can be prepared into an anti-tumor drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com