Photoinitiator based on cyclohexanedione-fused cyclodiene and synthesis method of photoinitiator
A technology of cyclohexanedione and photoinitiator, which is applied in the field of photoinitiator and its synthesis based on cyclohexanedione and cyclodiene, which can solve the problems of poor yellowing resistance and unpleasant smell, and achieve non-toxic and tasteless Yellowing, good initiation efficiency, simple synthesis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method of synthesizing a photoinitiator based on cyclohexidine and cycloneene, including the steps of:
[0029] The cyclohexidone and the diecedehyde (or ketone) compound were added to methanol than 1: 1: 1, and the mass fraction of 10% sodium hydroxide solution was dripped, protected from nitrogen gas, and reacted at 20 ° C for 3 h. The pale yellow solid was obtained, and then the resulting pale yellow solid was recrystallized to obtain a photoinitiator based on cyclohexadric albicadiene.
[0030] The amount of sodium hydroxide solution is 10% of cyclohexidone weight.
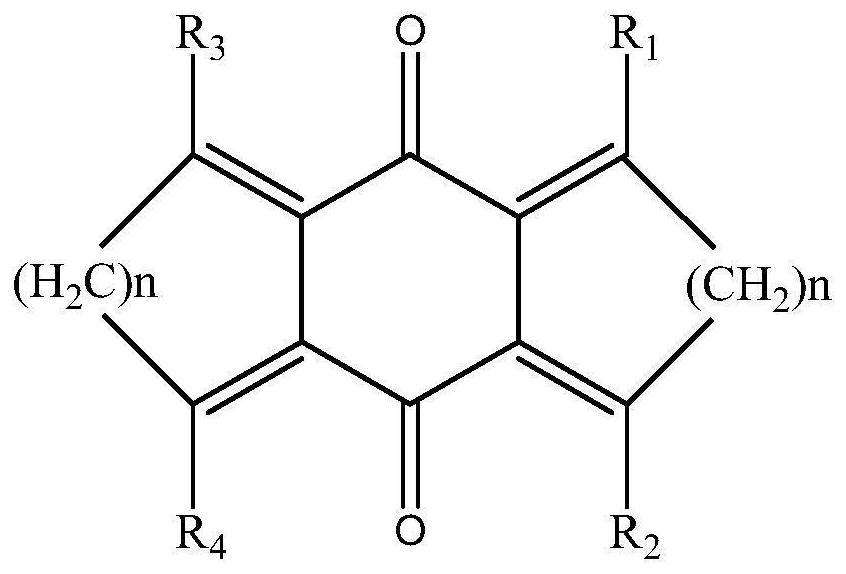
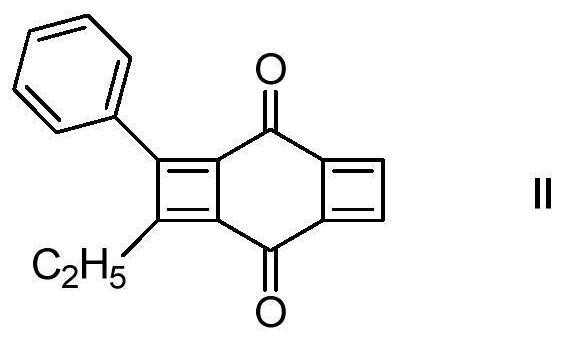
[0031] When N = 0, R1, R2, R3, R4 are independent representative of H, methyl, ethyl, phenyl, which has the structure of the formula (II), and the reaction process is as follows:
[0032]
[0033] A photoinitiator based on cyclohexadricarbide and has a structure shown in the following formula (II):
[0034]
Embodiment 2
[0036] A method of synthesizing a photoinitiator based on cyclohexidine and cycloneene, including the steps of:
[0037] The cyclohexidone and the diecedehyde (or ketone) compound were added to methanol than 1: 1: 1, and the mass fraction of 10% sodium hydroxide solution was dripped, protected from nitrogen gas, and reacted at 50 ° C for 5 h. The pale yellow solid was obtained, and then the resulting pale yellow solid was recrystallized to obtain a photoinitiator based on cyclohexadric albicadiene.
[0038] The amount of sodium hydroxide solution is 10% of cyclohexidone weight.
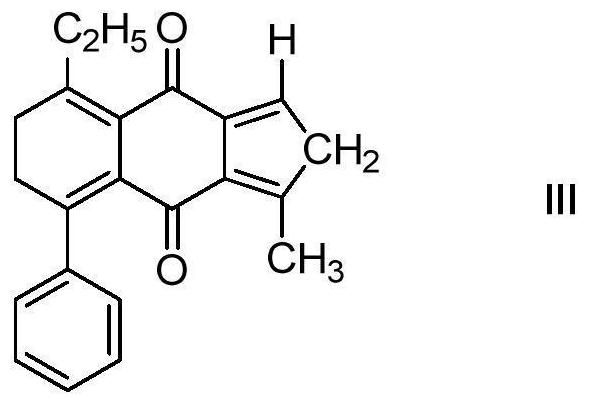
[0039] When N = 1, R1, R2, R3, R4 are independently representing H, methyl, ethyl, phenyl, the photoinitiator has the structure of the following formula (III), and the reaction process is as follows:
[0040]
[0041] A photoinitiator based on cyclohexidine and cycloneene having a structure shown in the following formula (III):
[0042]
Embodiment 3
[0044] A method of synthesizing a photoinitiator based on cyclohexidine and cycloneene, including the steps of:
[0045] The cyclohexidone and the diecedehyde (or ketone) compound were added to methanol than 1: 1: 1, and the mass fraction of 10% sodium hydroxide solution was dripped, protected from light, nitrogen gas protection, reacted at 100 ° C for 12h, The pale yellow solid was obtained, and then the resulting pale yellow solid was recrystallized to obtain a photoinitiator based on cyclohexadric albicadiene.
[0046] The amount of sodium hydroxide solution is 10% of cyclohexidone weight.
[0047] When N = 2, R1, R2, R3, R4 independently represents H, methyl, ethyl, phenyl, the photoinitiator has the structure of the following formula (III), and the reaction process is as follows:
[0048]
[0049] A photoinitiator based on cyclohexidine and cycloylene, has a structure shown in the following formula (IIII):
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Coating hardness | aaaaa | aaaaa |
| Coating hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com