Hesperetin and betaine eutectic substance B, and preparation method, composition and application thereof
A technology of betaine and hesperetin, which is applied in the field of medicine, can solve the problems of no similar or conflicting research content, no co-crystal formation of hesperetin-betaine, etc., and achieve significant solubility and dissolution rate advantages. The effect of good safety drug advantage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Hesperetin and Betaine Cocrystal B Preparation Method 1
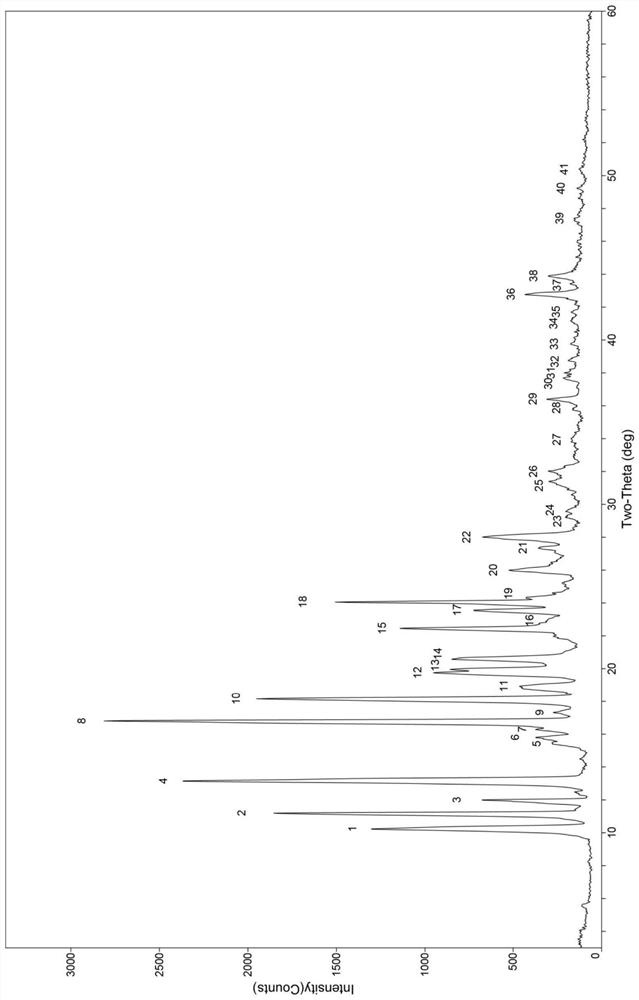
[0061] Take an appropriate amount of hesperetin and betaine, the molar ratio of the two is 1:1, add the sample into a suitable container by solvent suspension method at room temperature, add an appropriate amount of organic solvent, stir for an appropriate time at room temperature, and dissolve the obtained suspension Evaporation and drying of liquid solvent, natural drying by filtration or vacuum drying by filtration, see Table 3 for the condition parameters. Carry out powder X-ray diffraction analysis to it, its diffraction pattern and figure 1 Consistent, indicating that the obtained sample is cocrystal B of hesperetin and betaine.
[0062] Table 3 hesperetin and betaine cocrystal B preparation method 1 specific example
[0063]
Embodiment 2
[0065] Dissolution and release characteristics of hesperetin-betaine in vitro
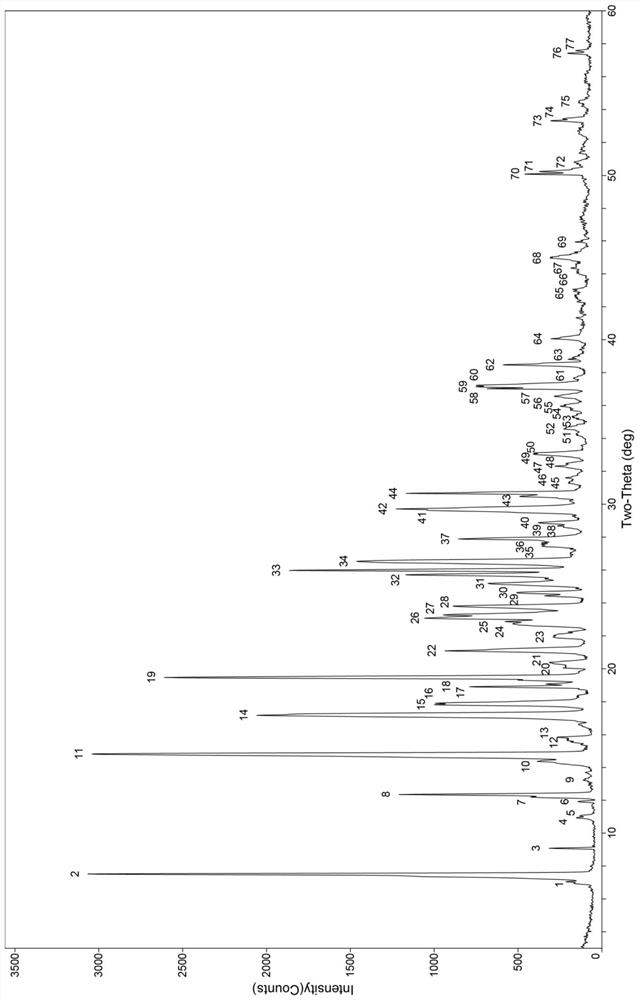
[0066] The solubility characteristics of hesperetin-betaine cocrystal B and hesperetin API in aqueous systems were investigated. The experiment was carried out with reference to the "Technical Guidelines for Dissolution Test of Ordinary Oral Solid Preparations", and the percentage of dissolution was calculated by the high performance liquid phase method and the external standard method. Take time as the abscissa, and the dissolution percentage as the ordinate to draw the dissolution curve respectively ( Figure 6 ). The data are shown in Table 4.
[0067] Detection conditions: detection system: Aligent 1200, chromatographic column: Agilent Eclipse XDB-C18 (4.6 × 150mm, 5μm); mobile phase: methanol-water (70:30, v / v); flow rate: 1mL min -1 ; Column temperature: 30°C; Detection wavelength: Hesperetin: 280nm; Injection volume: 10 μl.
[0068] Table 4 Dissolution Profile Data
[0069]
[0070] ...
Embodiment 3
[0072] Stability advantage of hesperetin and betaine cocrystal B
[0073] High temperature test: put the sample of hesperetin and betaine eutectic B into an open clean watch glass, place it at 60°C for 10 days, and take samples on the 0th day, the 5th day and the 10th day. Powder X-ray diffraction analysis was performed on the samples obtained at the above sampling points, and the results showed that the cocrystal B of hesperetin and betaine was stable under the test of high temperature influencing factors.
[0074] Illumination test: Put the hesperetin-betaine eutectic B sample in an open clean watch glass, put it in a light box equipped with a fluorescent lamp, and place it under the condition of 4500lx±500lx for 10 days, and then put it on the 0th day, the 1st day Samples were taken on days 5 and 10. Powder X-ray diffraction analysis was performed on the samples obtained at the above sampling points, and the results showed that the cocrystal B of hesperetin and betaine was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com