Anti-restenosis 3D printing self-expansion degradable intravascular stent and preparation method thereof
A 3D printing, vascular stent technology, applied in coating, medical science, surgery, etc., can solve the problems of low biocompatibility of implanted biomaterials, hindering the long-term clinical success of interventional therapy, etc. Good elasticity and short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
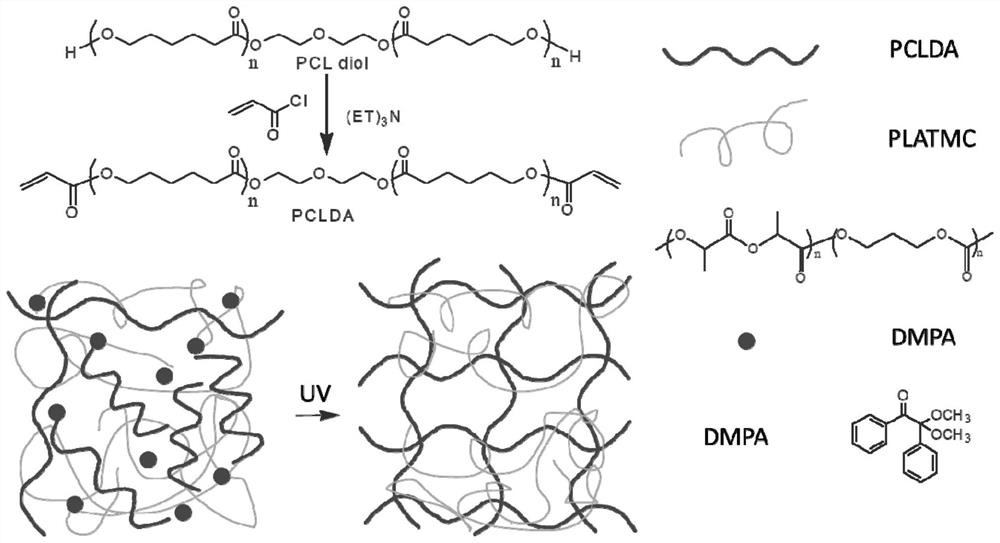
[0040] Such as Figure 5 As shown, a preparation method for anti-restenosis 3D printing self-expanding degradable vascular stent, comprising the following steps:
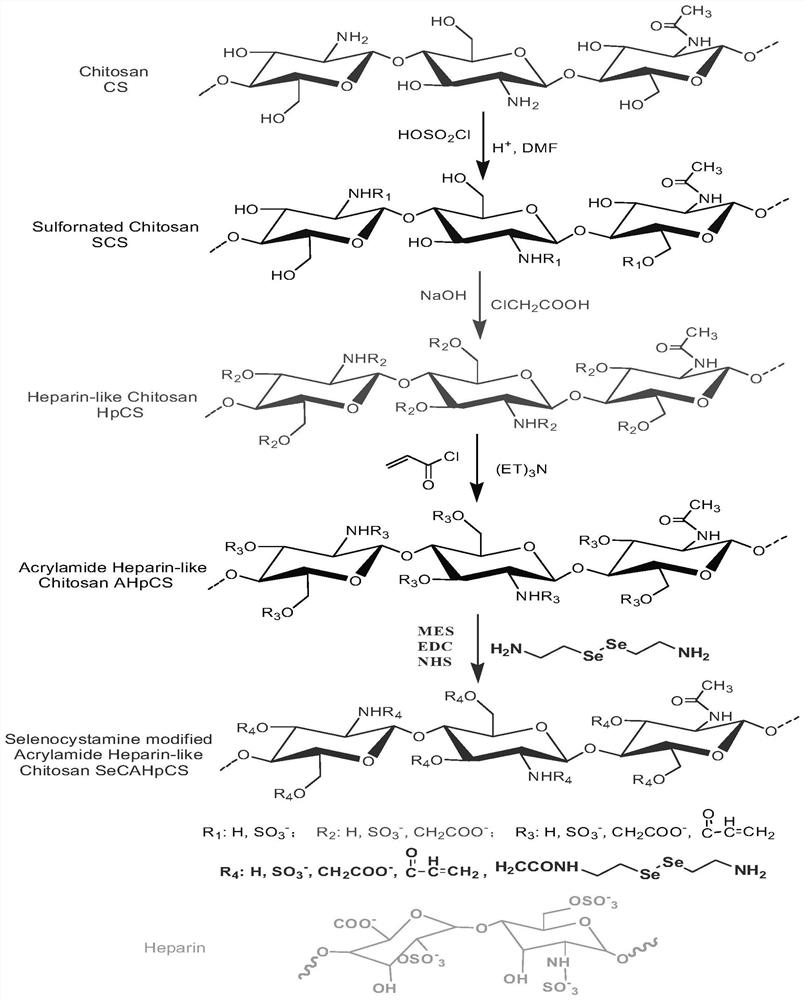
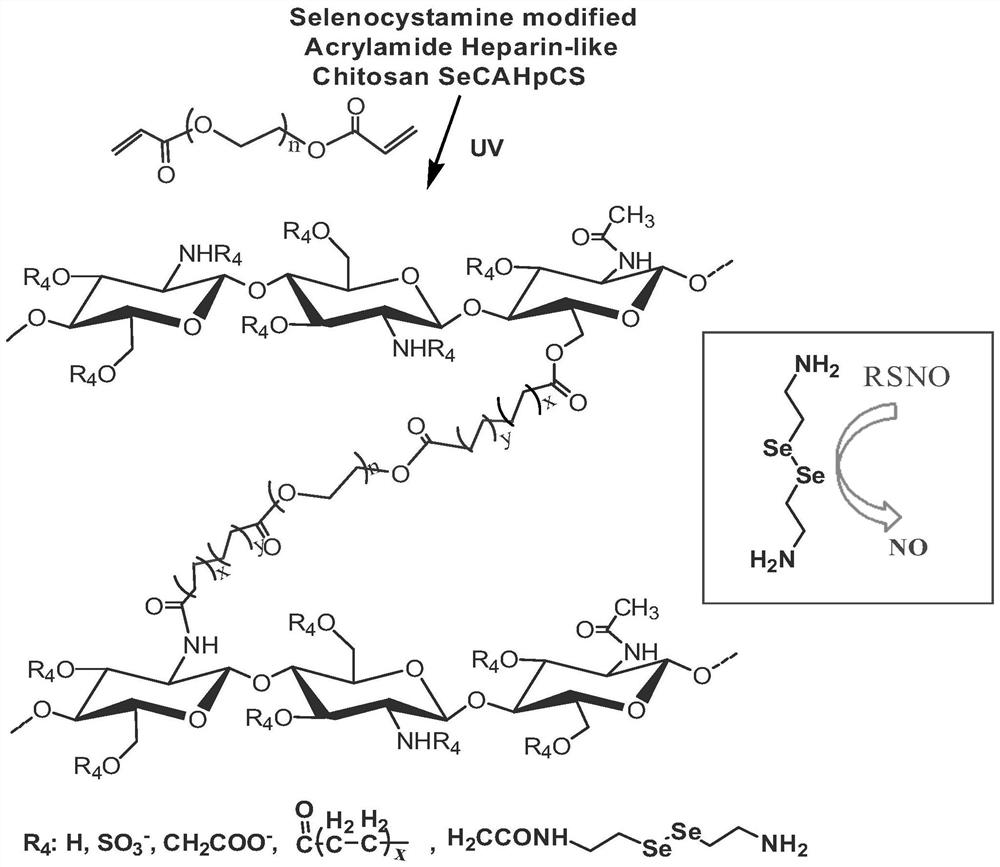
[0041] (1) Preparation of heparin-like / selenocystamine / acryloyl-modified chitosan: chitosan was mixed with chlorosulfonic acid, chloroacetic acid, and selenocystamine in sequence according to the molar ratio of 20:10:10:5:1 and acryloyl chloride reaction, wherein the molar mass of chitosan is calculated according to the molecular weight of the repeating unit of 161g / mol, and the hydroxyl and amino groups on the chitosan molecule are modified with sulfonic acid groups, carboxyl groups, selenocystamine groups and acryloyl groups, and regulated Grafting rate, prepared heparan / selenocystamine / acryloyl modified chitosan, the specific reaction formula is as follows figure 1 shown;
[0042] (2) Preparation of degradable hydrophilic layer 3D printing ink: the heparan / selenocystamine / acryloyl-modified chitosan obtained in...
Embodiment 2
[0046] Such as Figure 5 As shown, a preparation method for anti-restenosis 3D printing self-expanding degradable vascular stent, comprising the following steps:
[0047] (1) Preparation of heparin-like / selenocystamine / acryloyl-modified chitosan: Chitosan was mixed with chlorosulfonic acid, chloroacetic acid, and selenocystamine in sequence according to the molar ratio of 30:10:10:5:1 and acryloyl chloride reaction, wherein the molar mass of chitosan is calculated according to the molecular weight of the repeating unit of 161g / mol, and the hydroxyl and amino groups on the chitosan molecule are modified with sulfonic acid groups, carboxyl groups, selenocystamine groups and acryloyl groups, and regulated Grafting rate, prepared heparan / selenocystamine / acryloyl modified chitosan, the specific reaction formula is as follows figure 1 shown;
[0048] (2) Preparation of degradable hydrophilic layer 3D printing ink: the heparan / selenocystamine / acryloyl-modified chitosan obtained in...
Embodiment 3
[0052] Such as Figure 5 As shown, a preparation method for anti-restenosis 3D printing self-expanding degradable vascular stent, comprising the following steps:
[0053] (1) Preparation of heparin-like / selenocystamine / acryloyl-modified chitosan: Chitosan was sequentially mixed with chlorosulfonic acid, chloroacetic acid, and selenocystamine at a molar ratio of 15:10:10:5:1 and acryloyl chloride reaction, wherein the molar mass of chitosan is calculated according to the molecular weight of the repeating unit of 161g / mol, and the hydroxyl and amino groups on the chitosan molecule are modified with sulfonic acid groups, carboxyl groups, selenocystamine groups and acryloyl groups, and regulated Grafting rate, prepared heparan / selenocystamine / acryloyl modified chitosan, the specific reaction formula is as follows figure 1 shown;
[0054] (2) Preparation of degradable hydrophilic layer 3D printing ink: the heparan / selenocystamine / acryloyl-modified chitosan obtained in step (1), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com