High-value utilization method of sodium 2-mercaptoacetate in thionocarbamate production tail liquid
A technology of sodium thioglycolate and sodium thioacetate, which is applied in organic chemistry, solid separation, flotation, etc., can solve the problems that cannot completely solve the problem of 2-thioglycolate tail liquid accumulation and limited market, and achieve excellent extraction performance , Increase the effect of chelating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Preparation of sodium 2-mercaptoacetate during the preparation of O-isopropyl-N-ethylthiocarbamate (Z-200)
[0080] Add 9.5 parts of 2-chloroacetic acid with a purity of 99% into the reaction vessel, then add 30 parts of distilled water to completely dissolve the 2-chloroacetic acid, and add 5.9 parts of Na with a purity of 99.8% in batches 2 CO 3 When the pH of the solution is about 8.0 (±0.1), add 18.59 parts of sodium isopropylxanthate with a purity of 85.0%, stir magnetically while adding, and heat up to 80°C for 2 hours after the addition. Cool the reaction solution below 20°C, add 6.76 g of ethylamine aqueous solution with a purity of 68% to 72% dropwise with a constant pressure dropping funnel, stir magnetically while adding, raise the temperature to 70°C after the dropwise addition, and react for 1 hour , cooled to room temperature, and separated to obtain 14.38 parts of the Z-200 product as a light yellow oil in the upper layer, and 55.64 parts of a...
Embodiment 2
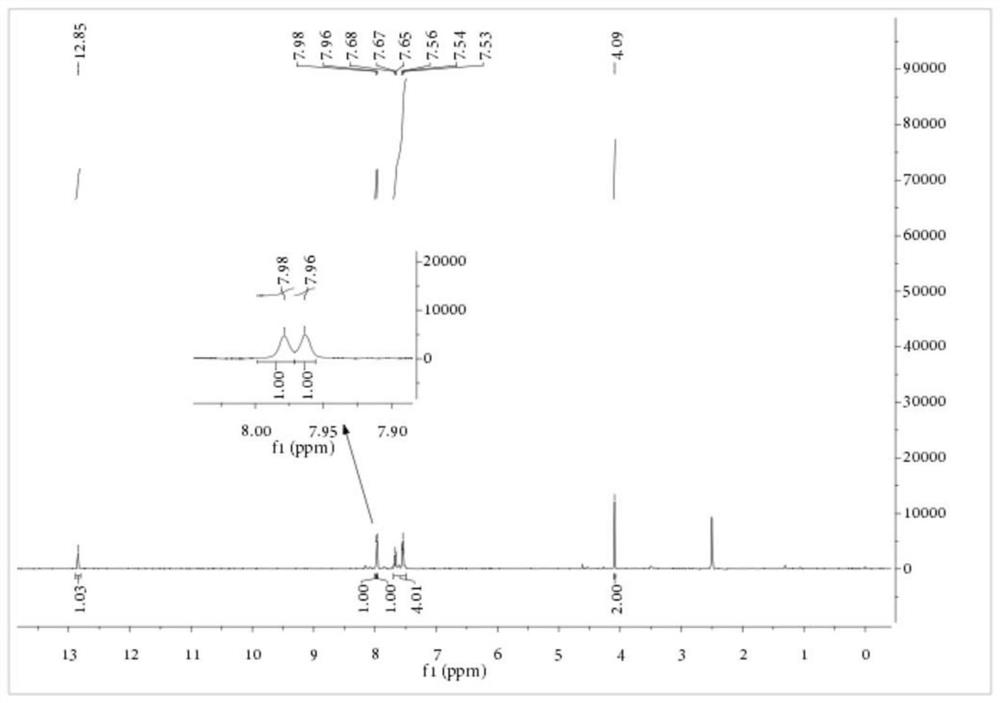
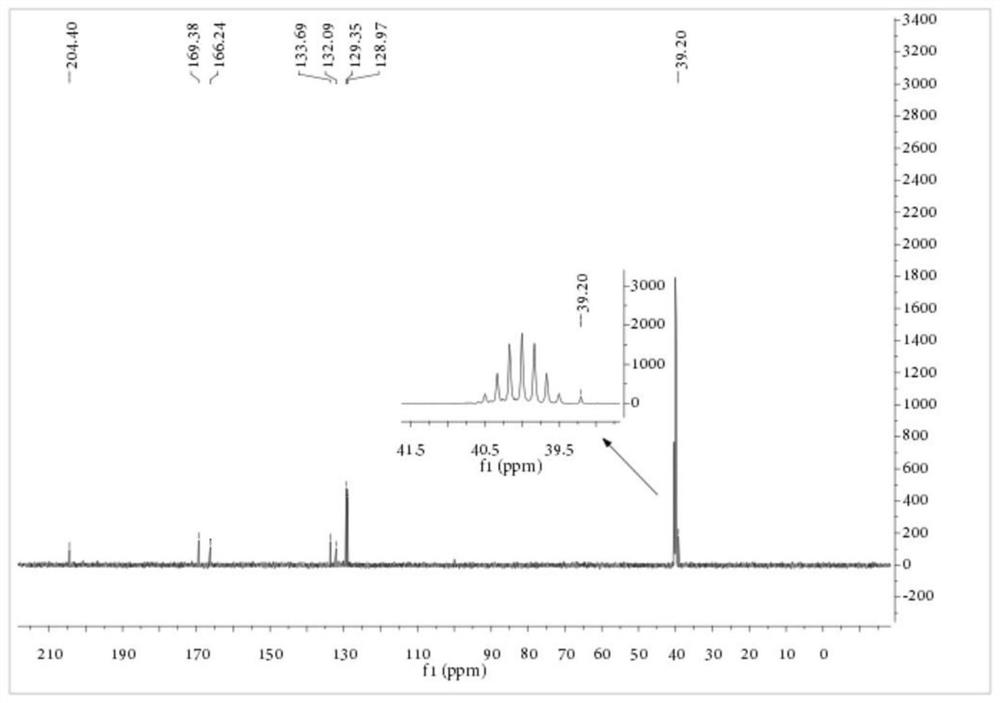
[0081]Embodiment 2: Preparation of S-carboxyethyl-N-benzoyl dithiocarbamate
[0082] Add 1.15 parts of PEG-400 with a purity of 99%, 11.83 parts of KSCN with a purity of 98.5% and 80 parts of dichloromethane with a purity of 99% into a 250mL three-necked flask, stir at 10°C for 10 minutes, and then add 13.63 A portion of benzoyl chloride with a purity of 99% was heated up to 25° C. for 3.5 hours. After the reaction, the salt was removed by filtration, the dichloromethane was recovered by rotary evaporation, and 55.64 parts of the aqueous solution of sodium 2-mercaptoacetate with a purity of 19.66% obtained in Example 1 were transferred to a reaction vessel, and the temperature was raised to 35° C. for 4 hours. After the reaction, add concentrated sulfuric acid to acidify, and filter to obtain a yellow solid that is the crude product of S-carboxyethyl-N-benzoyl dithiocarbamate. The product purity is 83.34%, based on the yield of sodium 2-mercaptoacetate was 85.21%. The crude ...
Embodiment 3
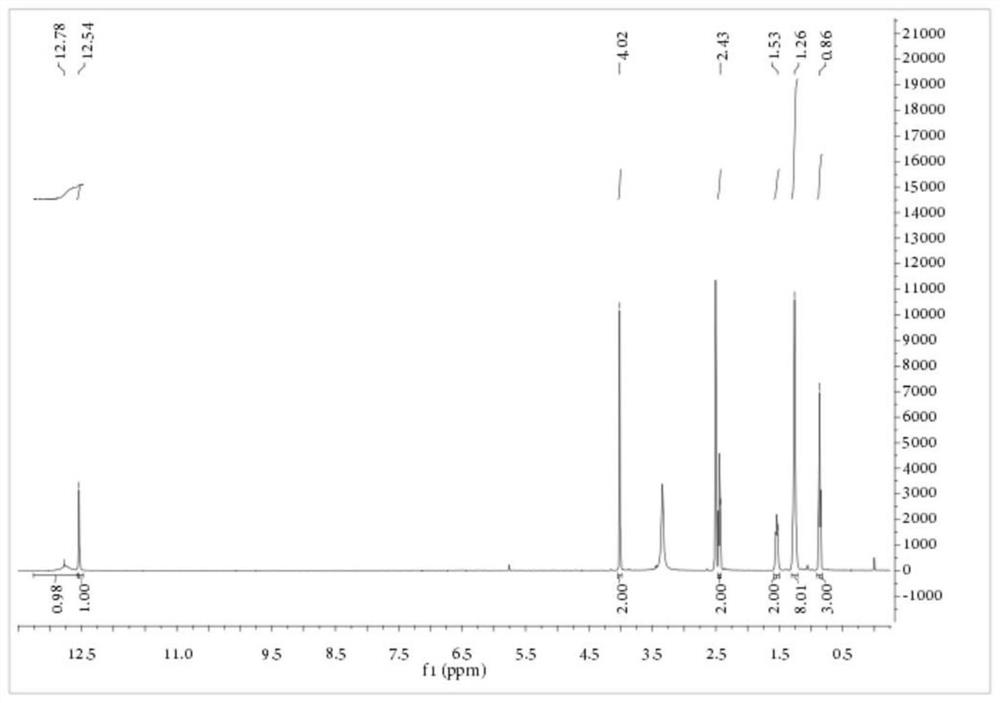
[0083] Embodiment 3: Preparation of S-carboxyethyl-N-octanoyl dithiocarbamate
[0084] Add 1.15 parts of PEG-400 with a purity of 99%, 11.83 parts of KSCN with a purity of 98.5% and 80 parts of dichloromethane with a purity of 99% into a 250mL three-necked flask, stir at 10°C for 10 minutes, and then add 15.77 A portion of octanoyl chloride with a purity of 99% was heated to 40° C. for 3.5 hours. After the reaction, the salt was removed by filtration, the dichloromethane was recovered by rotary evaporation, and 55.64 parts of the aqueous solution of sodium 2-mercaptoacetate with a purity of 19.66% obtained in Example 1 were transferred to a reaction vessel, and the temperature was raised to 35° C. for 4 hours. After the reaction, add concentrated sulfuric acid to acidify, and filter to obtain a yellow solid, which is the crude product of S-carboxyethyl-N-octanoyl dithiocarbamate. The product purity is 82.22%, and the yield based on 2-mercaptoacetate sodium is 83.92%. The cru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com