Co-production method of thionocarbamate and benzyl thioether acetic acid and application of the method
A technology of benzyl sulfide and thiourethane, applied in flotation, sulfide preparation, solid separation, etc., can solve the problems of difficult recycling of by-products, low economic benefits, etc., and achieve improved flotation recovery rate and atom utilization High efficiency and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: Preparation of O-isopropyl-S-benzyl xanthate
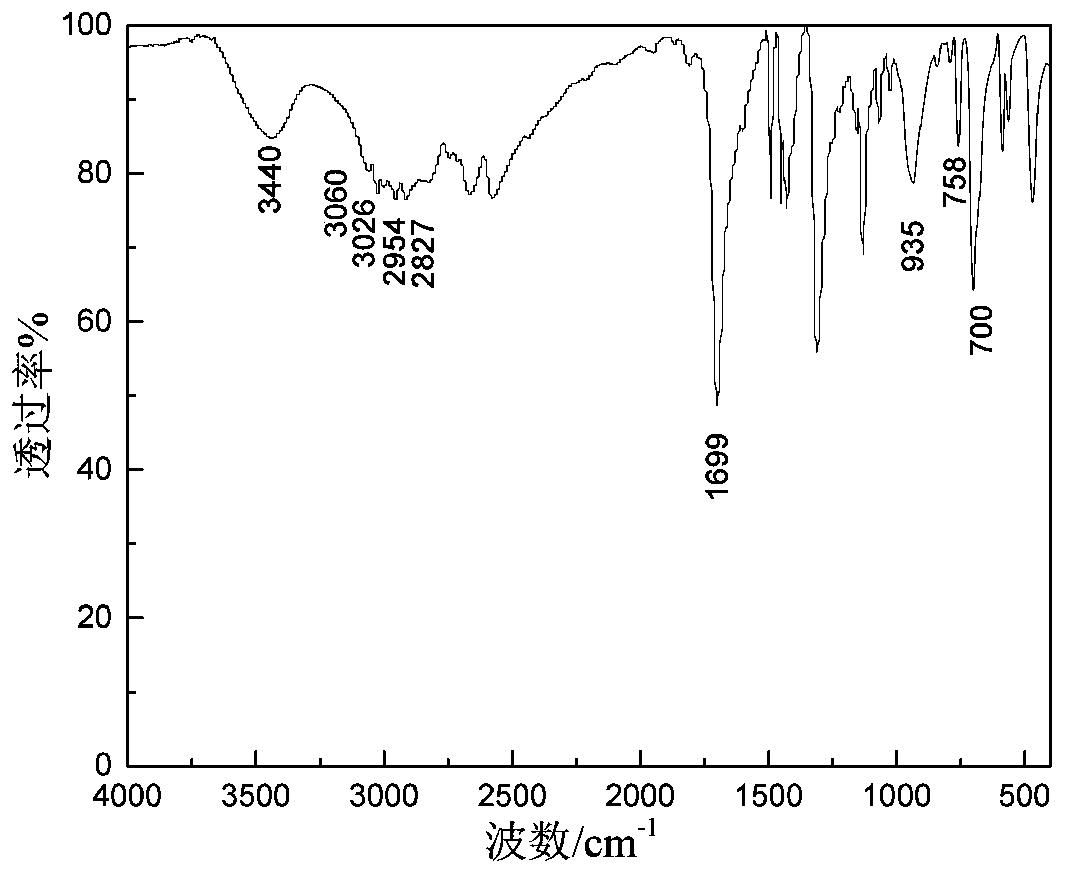
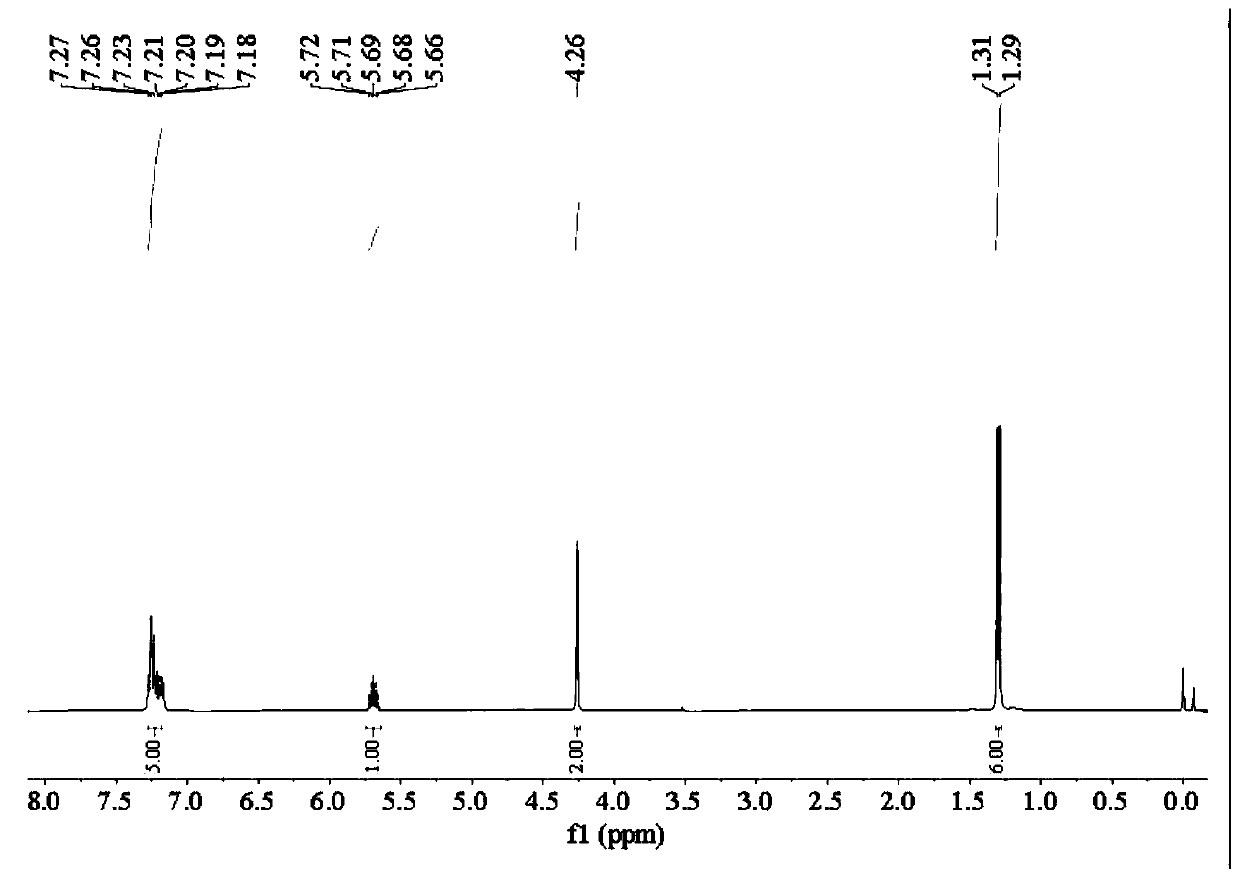
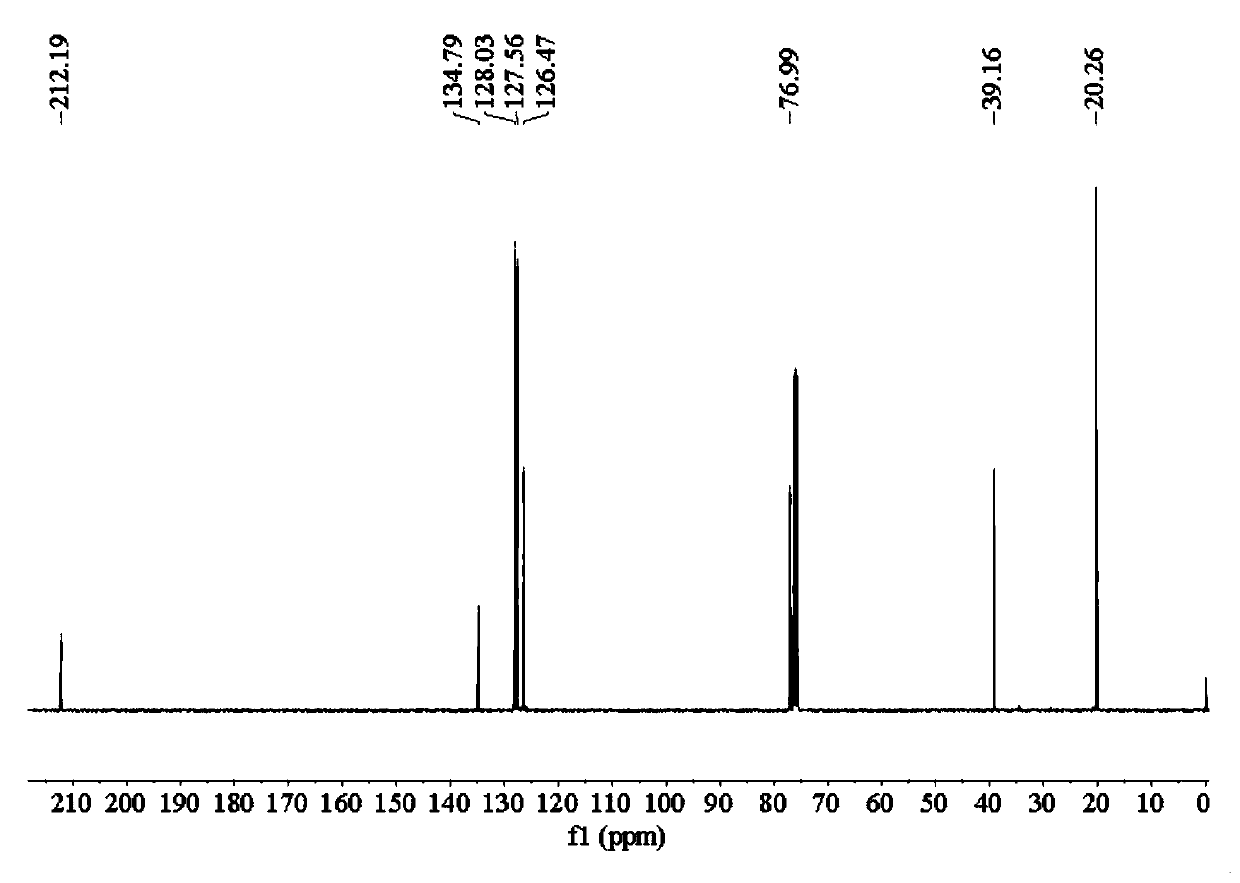
[0074] Add 20.45 parts of sodium isopropylxanthate with a purity of 85% into the reaction vessel, then add 30 parts of distilled water to completely dissolve the sodium isopropylxanthate, then add 12.79 parts of benzyl chloride with a purity of 99%, and heat up to React at 75°C for 4 hours, cool to room temperature, and separate to obtain 22.52 parts of O-isopropyl-S-benzyl xanthate product. The product purity is 97.44%, and the yield based on benzyl chloride is 97.10%.
Embodiment 2
[0075] Embodiment 2: the preparation of O-isopropyl-N-ethyl thiocarbamate (Z-200)
[0076] 22.52 parts of O-isopropyl-S-benzyl xanthate obtained in Example 1 with a purity of 97.44% were transferred to the reaction vessel, and 6.76g parts with a purity of 68 to 72 % ethylamine aqueous solution, magnetically stirred while adding, after the dropwise addition, the temperature was raised to 70°C, reacted for 1 hour, and after cooling to room temperature, 11.88 parts of sodium chloroacetate with a purity of 98% were added to the resulting mixed system. React for 2 hours at Celsius, cool to room temperature, and separate the liquids. The oil phase is 15.56 parts of Z-200, and the water phase is an aqueous solution of sodium benzyl sulfide acetate. Among them, Z-200 has a purity of 97.58% and a yield of 97.0%.
Embodiment 3
[0077] Embodiment 3: the preparation of benzyl tert-butoxyethyl xanthate
[0078] 4.82 parts of purity are 96% powdery sodium hydroxide to join in the reaction vessel, add 7.23 parts of distilled water, then add 13.13 parts of ethylene glycol tert-butyl ether with a purity of 99% in the bottle, and use constant pressure under mechanical stirring Add 8.46g of carbon disulfide dropwise into the dropping funnel, react at 25°C for 2 hours, add 25 parts of distilled water after the reaction is completed, then add 12.79 parts of benzyl chloride with a purity of 99%, heat up to 75°C for 4 hours, cool to room temperature, and separate the liquids to obtain 24.99 parts of O-tert-butoxyethyl-S-benzyl xanthate product. The product purity is 97.50%, and the yield based on benzyl chloride is 98.20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com