Production method of cimetidine
A production method and technology of cimetidine, which are applied in the field of preparation of cimetidine, can solve problems such as harm to human body and environment, high production cost, environmental pollution, etc., and achieve mild reaction conditions, little environmental pollution and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
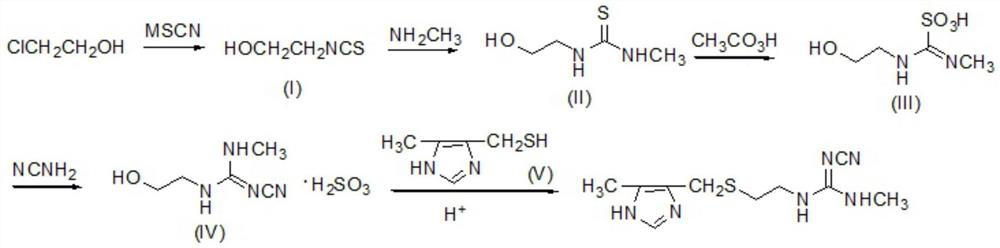
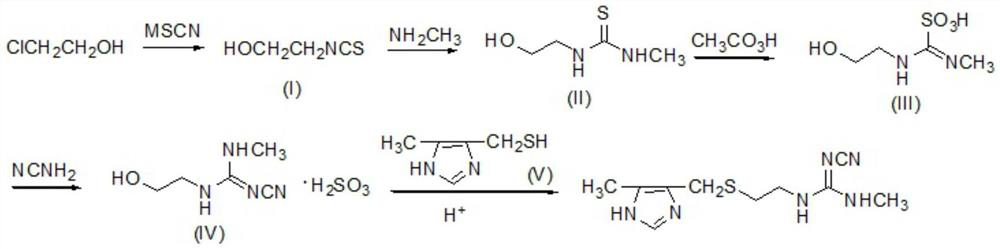
[0027] Dissolve 83.6Kg (1.1Kmol) NH4SCN in 500L water, add 1.45Kg (0.01Kmol) ammonium iodide, then add 80.5Kg (1Kmol) 2-chloroethanol, reflux for 3 hours, cool down to room temperature, stand still, and remove water layer, the oil layer was washed with water, dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain intermediate (I). Yield 82.3%. Elemental analysis: C3H5NOS, calculated: C, 34.93, H, 4.89, N, 13.58, S, 31.09; found: C, 34.90, H, 4.91, N, 13.61, S, 31.07. 1H NMR (400MHz, CDCl3) δ2.85 (br, s, 1H), 3.60 (m, 2H), 3.79 (m, 2H).
[0028] Dissolve 103Kg (1Kmol) of intermediate (I) in 350L of ethanol, add 116.3Kg of monomethylamine solution with a mass fraction of 40%, react at 50°C for 3h, evaporate to dryness under reduced pressure to obtain intermediate (II), and use the crude product directly react in the next step. Elemental Analysis: C4H10N2OS, Calculated: C, 35.80, H, 7.51, N, 20.87, S, 23.89; Found: C, 35.83, H, 7.52, N, 2...
Embodiment 2
[0033] Dissolve 85.1Kg (1.05Kmol) NaSCN in 500L water, add 4.5Kg (0.03Kmol) sodium iodide, then add 80.5Kg (1Kmol) 2-chloroethanol, reflux for 5h, cool down to room temperature, stand still, and remove water layer, the oil layer was washed with water, dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain intermediate (I). Yield 84.5%.
[0034]Dissolve 103Kg (1Kmol) of intermediate (I) in 350L of ethanol, add 155Kg of monomethylamine solution with a mass fraction of 40%, react at 60°C for 3h, and evaporate to dryness under reduced pressure to obtain intermediate (II). The crude product was directly used in the next reaction.
[0035] Add 134Kg (1Kmol) of intermediate (II) into 300L of acetic acid, stir, cool down to 8-10°C, add dropwise 1013Kg of peracetic acid solution with a mass fraction of 30%, control the rate of addition, make the temperature of the reaction system 20°C, add dropwise After completion, react at room temperature for...
Embodiment 3
[0039] Dissolve 116.4Kg (1.2Kmol) KSCN in 500L water, add 0.97Kg (0.02Kmol) potassium iodide, then add 80.5Kg (1Kmol) 2-chloroethanol, reflux for 3 hours, lower to room temperature, let stand, and separate the water layer, The oil layer was washed with water, dried over anhydrous sodium sulfate, filtered, and distilled under reduced pressure to obtain intermediate (I). Yield 85.3%.
[0040] Dissolve 103Kg (1Kmol) of intermediate (I) in 400L of ethanol, add 116.2Kg of monomethylamine solution with a mass fraction of 40%, react at 55°C for 2h, and evaporate to dryness under reduced pressure to obtain intermediate (II). The crude product was directly used in the next reaction.
[0041] Add 134Kg (1Kmol) of intermediate (II) into 350L of acetic acid, stir, cool down to 8-10°C, add dropwise 760Kg of peracetic acid solution with a mass fraction of 35%, control the rate of addition, make the temperature of the reaction system 15°C, add dropwise After completion, react at room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com