Application of HPA in preparation of medicine for treating non-alcoholic fatty liver disease
A fatty liver disease, non-alcoholic technology, applied in the medical field, to achieve the effect of reducing inflammatory response, low price, and reducing economic burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
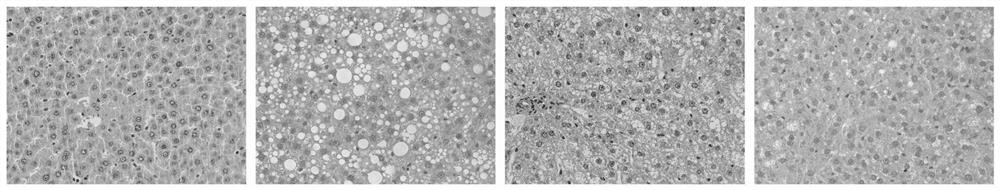
[0039] Example 1: Observation and research on rat liver tissue morphology and pathology after PHA treatment of NAFLD rats
[0040] The experimental animals used in the experiment are SD rats. The rats are all male and weigh 180-200g. They are provided by the Experimental Animal Center of Kunming Medical University. The rats are kept in isolation with 12 large cages and 6 small cages. The feeding is conventional Feed, food and water ad libitum;
[0041] The experimental drug p-hydroxyphenylethyl anisate (HPA) was provided by Japan Co., Ltd.; high-density lipoprotein cholesterol (HDL-C), low high-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) kits; interleukin-1α (IL-1α), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α ) kit; total superoxide dismutase (T-SOD), malondialdehyde (MDA) and glutathione peroxidase (GSH-PX) and other related Elisa kits were purchased from ...
Embodiment 2
[0044] Example 2: Study on the improvement of rat liver function and lipid metabolism after HA treatment of NAFLD rats
[0045] The animals and experimental medicines used in this experiment are the same as in Example 1, and related kits such as AST, ALT, HDL-C, LDL-C, TG and TC were purchased from Bio-Swamp Company;
[0046] In the experiment, rat modeling and treatment were identical with the method adopted in Example 1;
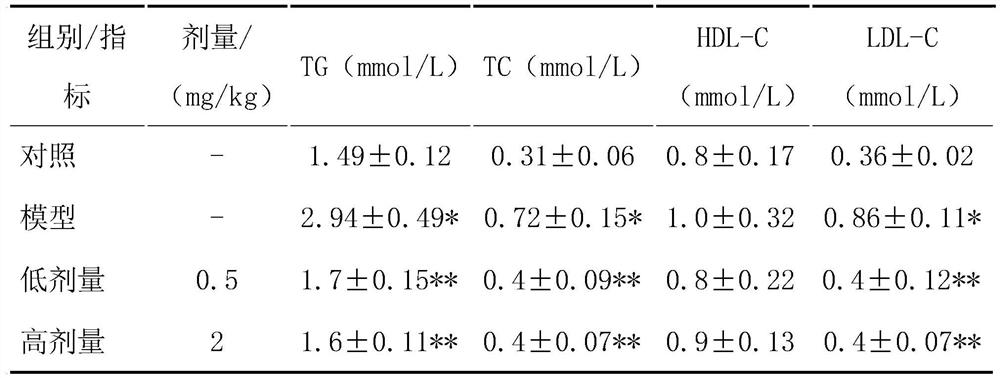
[0047] Detect 6 indexes including AST, ALT, HDL-C, LDL-C, TG and TC in rat serum with corresponding kits;
[0048] Compared with the blank control group, the serum AST and ALT, serum and liver HDL-C, LDL-C, TG, and TC in the model group were all significantly increased, and the above indicators in each treatment group were significantly lower than those in the model group (AST: model group 69.28±8.94mmol / L, HPA low-dose treatment group 74.48±9.08mmol / L, HPA high-dose treatment group 76.21±7.14mmol / L, blank control group 69.28±8.94mmol / L; ALT: model group ...
Embodiment 3
[0053] Example 3: Study on Antioxidant Capacity Content in Rat Serum after HPA Treated NAFLD Rats
[0054] The animals and experimental drugs used in this experiment are the same as in Example 1, and related kits such as SOD, MDA, GSH, and CAT were purchased from Nanjing Institute of Bioengineering;
[0055] In the experiment, rat modeling and treatment were identical with the method adopted in Example 1;
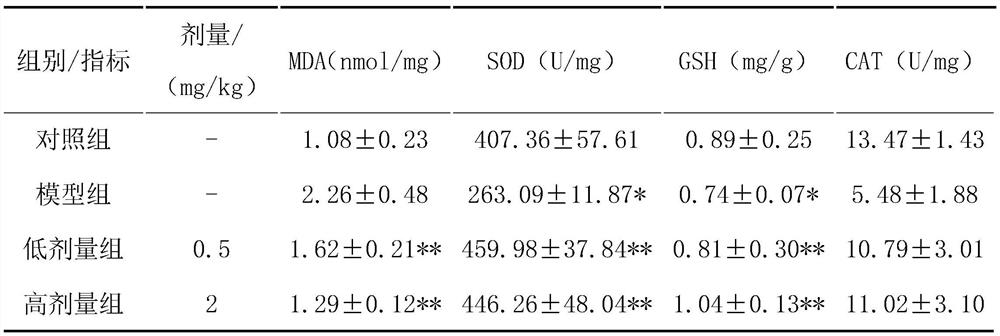
[0056] Use corresponding kits to detect the four indicators of rat serum SOD, MDA, GSH, and CAT;
[0057] Compared with the model group, the HPA treatment group has been significantly improved. After the test, the four indicators p<0.05, suggesting that HPA treatment of NAFLD rats can significantly improve the antioxidant capacity of the rats (SOD: model group 263.09±11.87 U / mg, HPA low-dose treatment group 459.98±37.84U / mg, HPA high-dose treatment group 446.26±48.04U / mg, blank control group 407.36±57.61U / mg; MDA: model group 2.26±0.48nmol / mg, HPA Low-dose treatment group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com