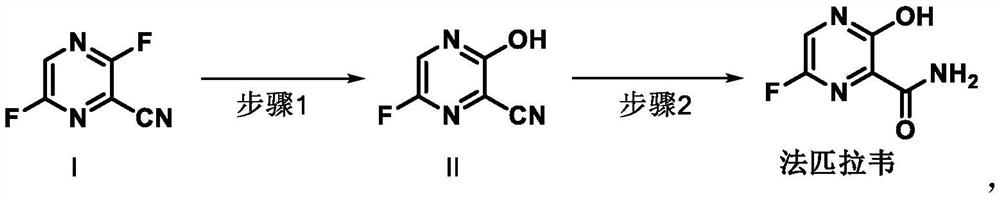
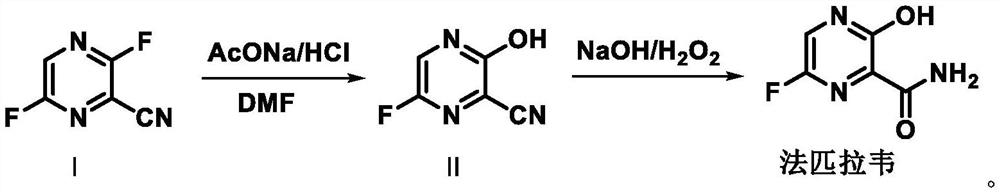
Preparation method of favipiravir
A technology of favipiravir and reaction solution, which is applied in the field of drug synthesis, can solve the problems of cost, increased processing difficulty, heterogeneous reaction, etc., and achieve the effect of reducing post-processing steps, avoiding drug safety, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 Favipiravir
[0050]
[0051] Add 5.0 g of 3,6-difluoro-2-cyanopyrazine and 10 ml of dimethyl sulfoxide into the reaction flask, and add aqueous sodium acetate solution (5.1 g of anhydrous sodium acetate dissolved in 20 ml of water) under stirring conditions. Heat to 50-60°C and stir until the reaction is complete. The reaction solution was filtered, and the filter cake was rinsed with 20 ml of purified water. Add dropwise sodium hydroxide aqueous solution (4.1g sodium hydroxide dissolved in 20ml water) to the filtrate under stirring condition. After the dropwise addition, add 10ml water and 5ml hydrogen peroxide solution dropwise. React and stir until the reaction is complete. Cool down to 20-30°C, add concentrated hydrochloric acid dropwise to adjust the pH to 3.0. The temperature was lowered to crystallize, filtered, and the filter cake was rinsed with purified water and dried to obtain 4.73 g of favipiravir with a yield of 85.0%...
Embodiment 2
[0052] The preparation of embodiment 2 Favipiravir
[0053]
[0054] Add 10.0 g of 3,6-difluoro-2-cyanopyrazine and 15 ml of dimethyl sulfoxide into the reaction flask, and add aqueous sodium acetate solution (17.5 g of anhydrous sodium acetate dissolved in 30 ml of water) under stirring conditions. Heat to 65°C and stir until the reaction is complete. Add dropwise sodium hydroxide aqueous solution (5.7g sodium hydroxide dissolved in 15ml water) to the reaction solution under stirring. After the dropwise addition, add 50ml of water, and dropwise add 15ml of hydrogen peroxide aqueous solution at 65°C. After the dropwise addition, stir until the reaction is complete. Cool down to 20-30°C, add concentrated hydrochloric acid dropwise to adjust the pH to 4.0. The temperature was lowered to crystallize, filtered, and the filter cake was rinsed with purified water and dried to obtain 8.1 g of favipiravir with a yield of 82.3% and a purity of 99.55%.
Embodiment 3
[0055] The preparation of embodiment 3 Favipiravir
[0056]
[0057] Add 20.0 g of 3,6-difluoro-2-cyanopyrazine and 20 ml of dimethyl sulfoxide into the reaction flask, and add aqueous sodium acetate solution (23.1 g of anhydrous sodium acetate dissolved in 80 ml of water) under stirring conditions. Heat to 50-60°C and stir until the reaction is complete. The reaction solution was filtered, and the filter cake was rinsed with 20 ml of purified water. Add aqueous sodium hydroxide solution (16.5g sodium hydroxide dissolved in 80ml water) dropwise to the filtrate under stirring condition, after the dropwise addition, add 40ml water, add 25ml hydrogen peroxide aqueous solution dropwise at 50-60°C, after the dropwise addition , and stir until the reaction is complete. Cool down to 20-30°C, add concentrated hydrochloric acid dropwise to adjust the pH to 3.0. The temperature was lowered to crystallize, filtered, and the filter cake was rinsed with purified water and dried to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com