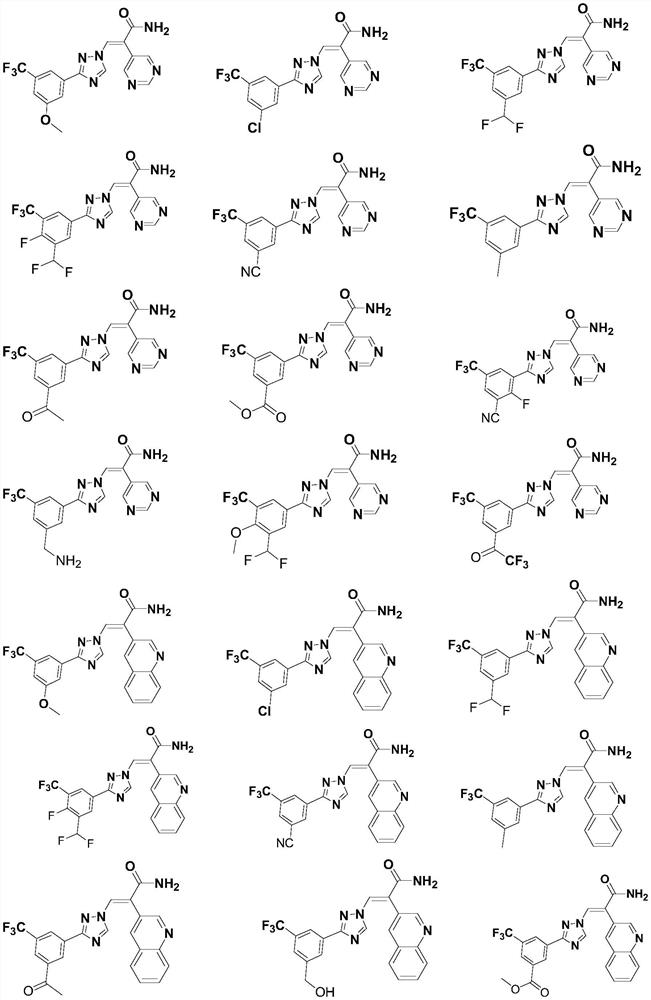
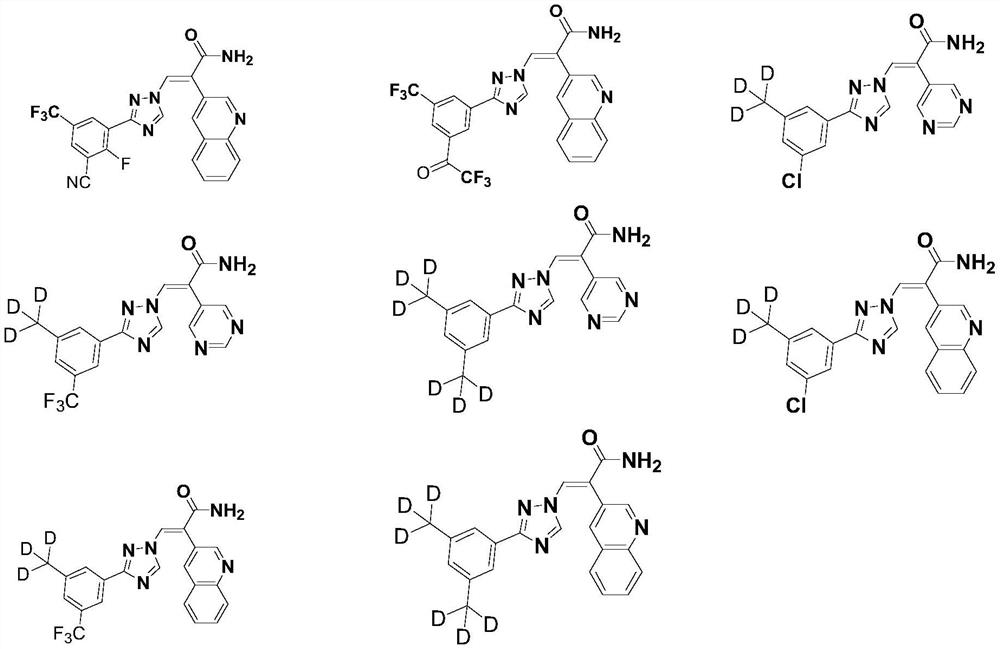
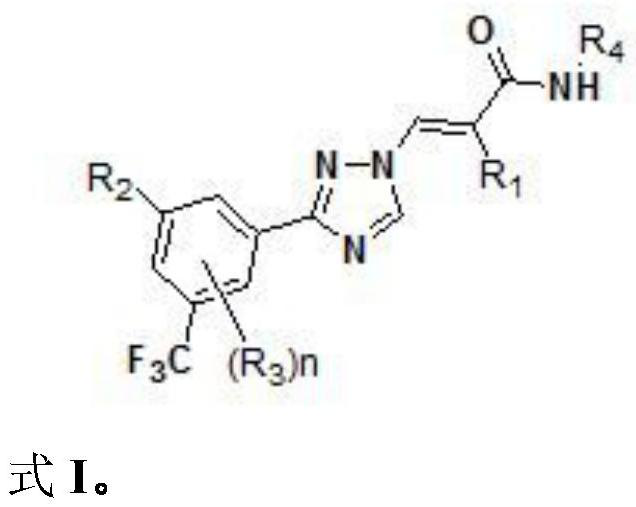
Five-membered nitrogen azole heterocyclic derivative as well as preparation method and application thereof
A technology of drugs and compounds, applied in the field of medicine, can solve problems such as high blood-brain barrier penetration and toxicity, and achieve good bioavailability, good cell activity, and high oral absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Step 1.1: Preparation of compound 1
[0020]
[0021] 3-Bromo-1,2,4-triazole (4.44g, 30.0mmol) was dissolved in 30ml of DMF, triethylenediamine (6.73g, 60.0mmol) was added, and stirred at room temperature for 30 minutes. Then (2Z)-isopropyl-3-iodoprop-2-enoate (7.92 g, 33.0 mmol) was added and stirred at room temperature for 1 h. The mixture was poured into 100ml ice water and the aqueous phase was extracted with ethyl acetate (3x50ml). The combined organic phases were washed twice with 50 ml of brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product. Column chromatography gave the target product (6.25 g, 24.0 mmol, 80% yield) as an off-white solid.
[0022] t R =1.222min, MS(ESI)m / z=259.8,261.7[M+H] + .
[0023] Step 1.2: Preparation of compound 2
[0024]
[0025] Dissolve (Z)-3-(3-bromo-1H-1,2,4-triazol-1-yl)isopropylacrylate (3.6g, 13.8mmol) in dichloromethane (50ml) at 0°C Bromine (4.41 g, ...
Embodiment 2
[0056] Embodiment 2: the preparation of compound P-B1
[0057] Preparation of intermediate Int-2
[0058] Step 1.1: Preparation of compound 1
[0059]
[0060] 3-Bromo-1,2,4-triazole (4.44g, 30.0mmol) was dissolved in 30ml of DMF, triethylenediamine (6.73g, 60.0mmol) was added, and stirred at room temperature for 30 minutes. Then (2Z)-isopropyl-3-iodoprop-2-enoate (7.92 g, 33.0 mmol) was added and stirred at room temperature for 1 h. The mixture was poured into 100ml ice water and the aqueous phase was extracted with ethyl acetate (3x50ml). The combined organic phases were washed twice with 50 ml of brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product. Column chromatography gave the target product (6.25 g, 24.0 mmol, 80% yield) as an off-white solid.
[0061] t R =1.222min, MS(ESI)m / z=259.8,261.7[M+H] + .
[0062] Step 1.2: Preparation of compound 2
[0063]
[0064] Dissolve (Z)-3-(3-bromo-1H-1,2,...
Embodiment 3
[0093] Embodiment 3: cell anti-proliferation activity (IC 50 )test
[0094] To prepare cells, add 100uL cells to a 96-well plate and incubate overnight at 37°C. The next day, the cells were diluted with DMSO to prepare the desired final concentration. Dilute the sample 3 times to the required concentration, add 50uL sample to the cell culture medium, 37°C, 5% CO 2 Incubate for 72h. After equilibrating to room temperature, pipette 40uL Add Reagent to the well plate, mix well, and incubate at room temperature for 60 minutes before detection.
[0095] [Table 3] Determination results of compounds
[0096]
[0097]
[0098] In Table 3: A≤30nM, 30nM1μM, NT: not tested
[0099] It can be seen from the data in the above table that the compounds of the present invention have stronger antiproliferative activity on tumor cells (human multiple myeloma cells (MM.1S)), such as compounds P-A1 and P-B1 on human multiple myeloma cells MM. Compared with the positive drug KPT-8602,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com