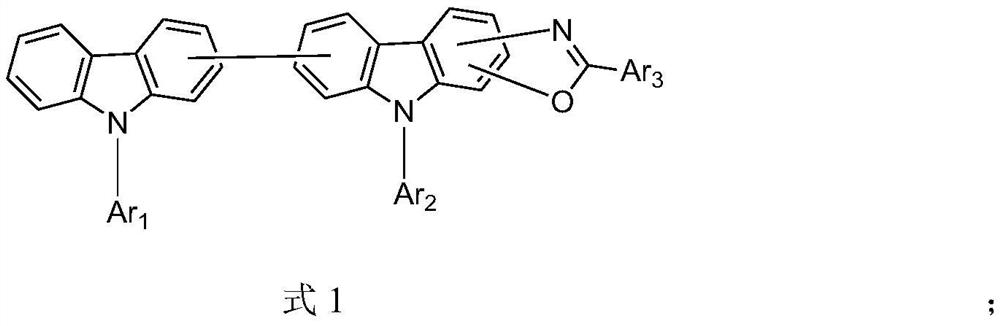
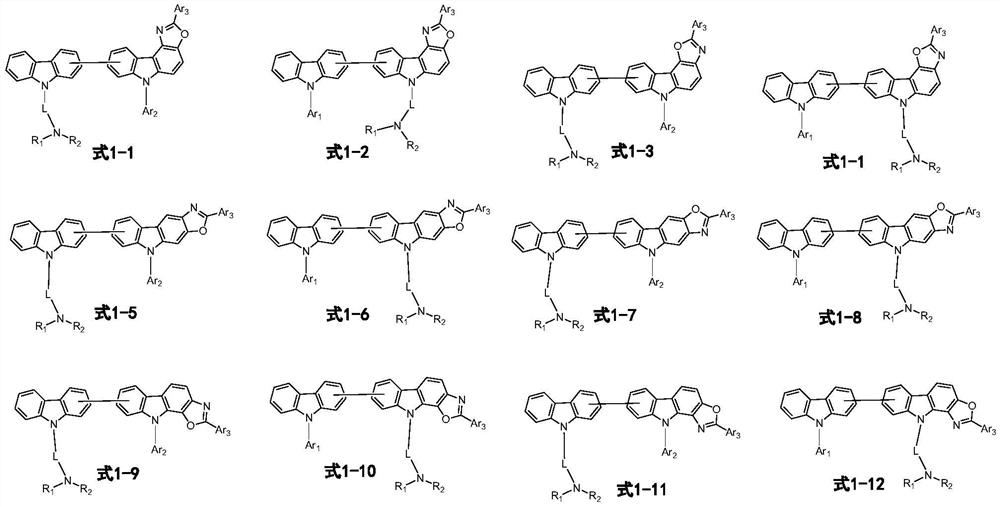
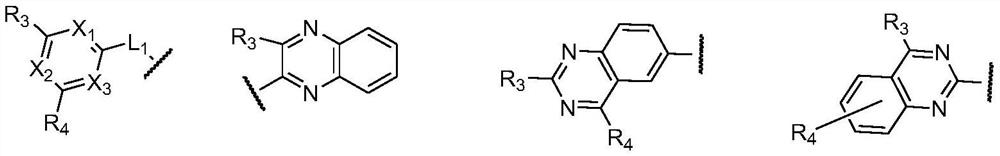
Organic compound and organic light-emitting device using same
A technology of organic light-emitting devices and organic compounds, applied in organic chemistry, light-emitting materials, electric solid-state devices, etc., can solve the problems of lack of materials and achieve the effects of easy-to-obtain raw materials, reduce vibration energy loss, and high-efficiency light-emitting performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Synthesis of Compound 1
[0064] 1, synthesis of intermediate 1-1
[0065]
[0066] Intermediates 1-1-2 (22.30 g, 61.4 mm), A (27.90 g, 61.4 mm), tetra (triphenylphosphine) palladium (5 mt), potassium carbonate (5) 17.0 g, 122.8 mm, 1,4-dioxane (200 ml) and water (50 ml). The reaction system was tapered to 80 degrees Celsius, and the reaction was 12 hours under nitrogen. After the reaction was completed, the reaction solution was cooled to room temperature, extracted with o-dichlorobenzene and water. The organic layer was dried over anhydrous magnesium sulfate, concentrated, and the crude crystalline was obtained by silica gel column to obtain intermediate 1-1 (30.63 g, yield 72%). LC-MS: M / Z692.26 (M + H) + .
[0067] 2, the synthesis of Compound 1
[0068]
[0069] The intermediate 1-1 (13.86 g, 20 millimorate), B (5.05 g, 21.0 mm), B (5.05 g, 21.0 mm), B (dibenzyl acetone) di palladium (4 marthyme), three t-but Cylin (8%), potassium t-butoxide (3.8 g, 33...
Embodiment 2
[0070] Example 2: Synthesis of Compound 13
[0071] 1, synthesis of intermediate 13-1
[0072]
[0073] Intermediate 1-1-2 (7.24 g, 20 mm), 4-iodine-N, N-diphenylbenzoamine (7.80 grams, 21.0 mm Mochi), 3 (Siya) in a 250 ml of the three flask Benzylcycecetone) Dipalide (4)%), tri-butylphosphine (8% by weight), potassium tert-butoxide (3.8 g, 33.6 mm) and o-xylene (80 ml). The reaction system was tapered to 120 degrees Celsius and the reaction was reacted under nitrogen for 12 hours. After completion, the reaction solution was cooled to room temperature, extracted with o-chlorophenyl and water. The organic layer was dried over anhydrous magnesium sulfate, concentrated, and the crystal obtained was crystallized to the compound 13-1 (10.31 g, yield 85%). LC-MS: M / Z605.11 (M + H) + .
[0074] 2, synthesis of intermediate 13-2
[0075]
[0076] Intermediate 13-1 (37.24.24.00 g, 61.4 mm), carbazole-3-boric acid (12.96 g, 61.4 mm), tetra (triphenylphosphine) palladium (5) %), Potass...
Embodiment 3
[0080] Example 3: Synthesis of Compound 26
[0081] The compound 26 is synthesized from the method of Example 1 to obtain compound 26 (10.36 g, yield of 70%). LC-MS: M / Z972.36 (M + H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com