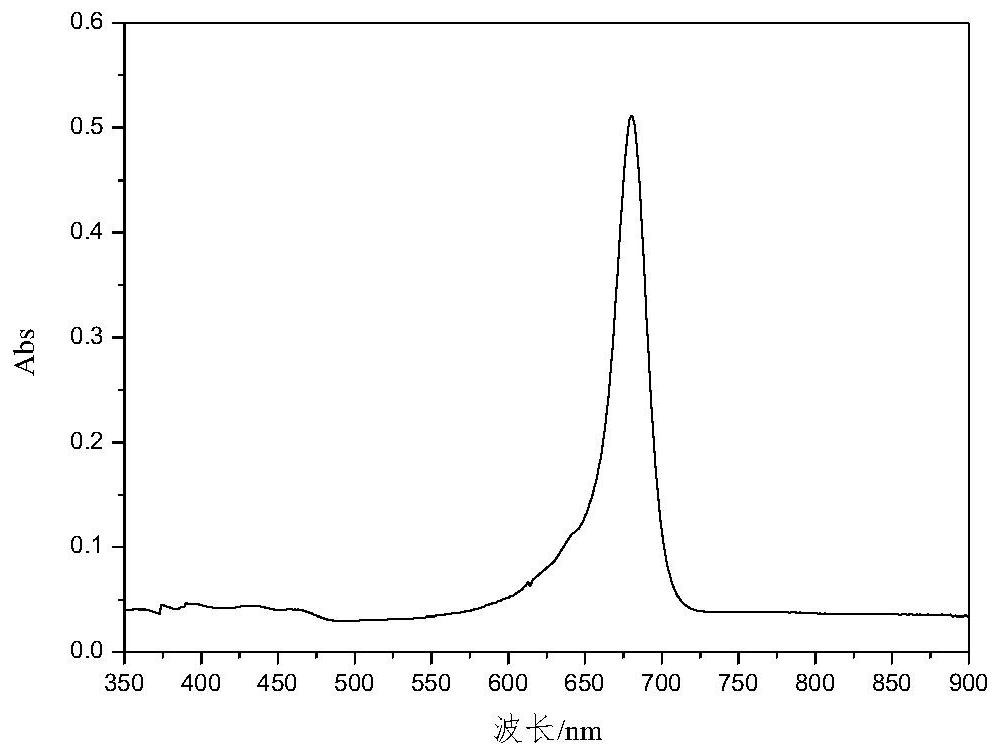
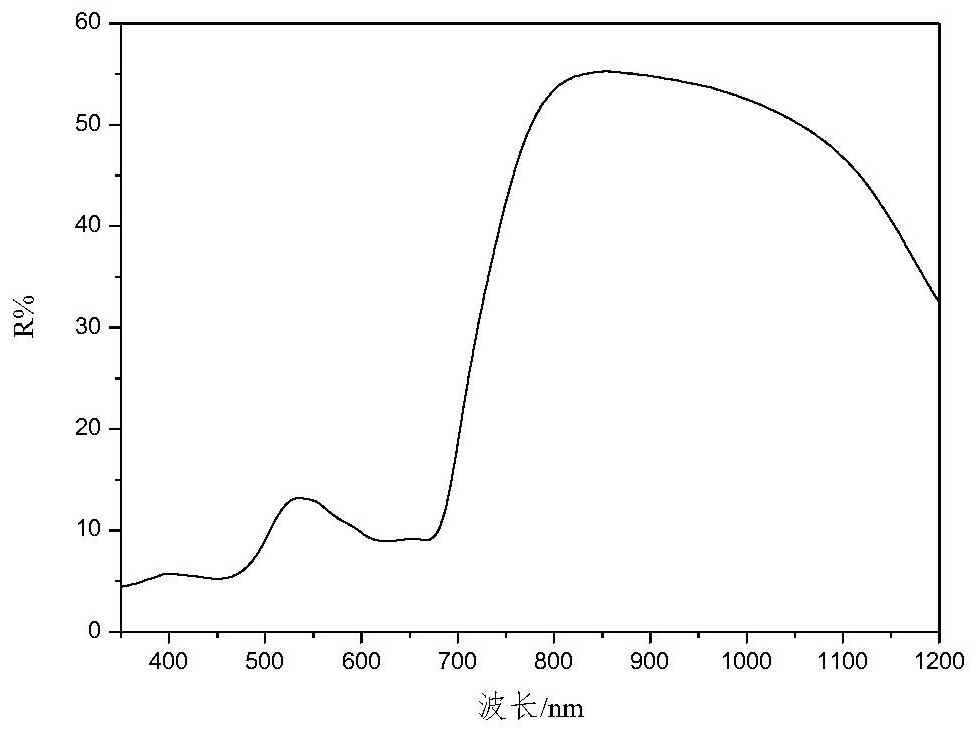
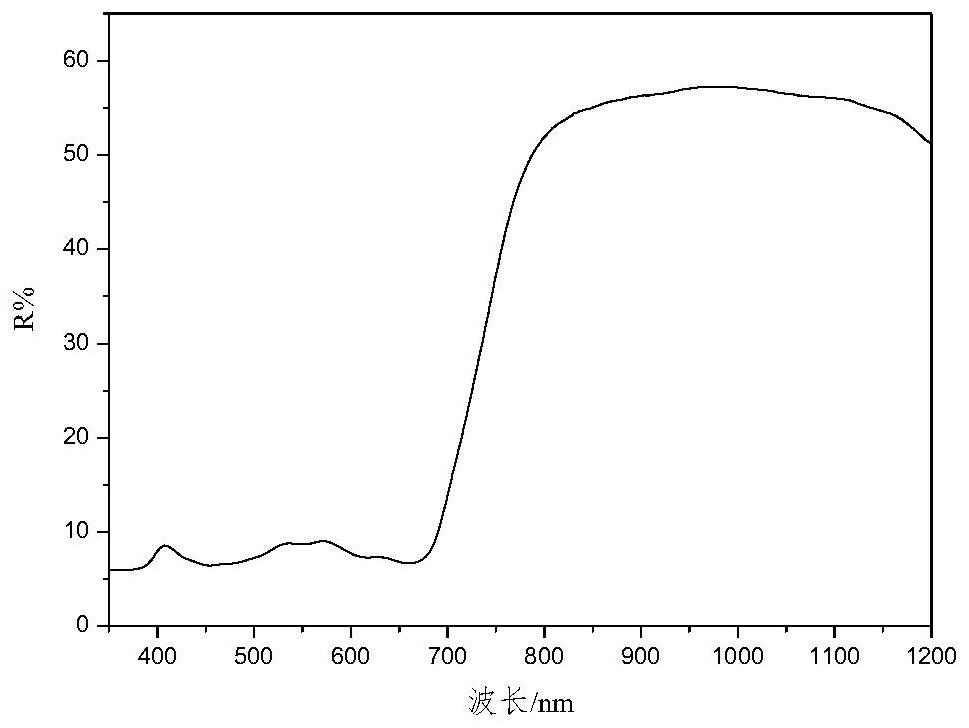
High-selectivity red light absorption heptamethine cyanine dye as well as synthesis method and application thereof
A heptamethine, high-selectivity technology, applied in the direction of methine/polymethine dyes, organic dyes, chemical instruments and methods, etc., can solve the problems of poor photostability and achieve less by-products and good red light selection Absorption performance, the effect of good selective absorption characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention also proposes a method for synthesizing the heptamethine cyanine dye with high selective red light absorption as described above, comprising the following steps:
[0028] S1: Weigh 2,3,3-trimethyl-3H-indole-5 sulfonic acid potassium salt and p-benyl bromobenzoic acid in a molar ratio of 1:(1~1.05), dissolve them in acetonitrile, and inert Under the protection of the atmosphere, the reflux reaction was carried out to obtain the intermediate product Ⅰ;
[0029] S2: Weigh the intermediate product I, diethylamine, N,N'-dicyclohexylcarboimide and 4-dimethylaminopyridine at a molar ratio of 1:1.2:0.2:0.5, and weigh the intermediate product I Place diethylamine in a three-necked flask containing dichloromethane, then add N,N'-dicyclohexylcarboimide and 4-dimethylaminopyridine to fully react to obtain intermediate product II;
[0030] S3: Weigh the intermediate product II and 2-chloro-1-formyl-3-hydroxymethylenecyclohexene in a molar ratio of 1:(1-1.05), ...
Embodiment 1
[0068] This embodiment provides a heptamethine cyanine dye with highly selective red light absorption, the structural formula of the heptamethine cyanine dye is:
[0069]
[0070] This embodiment also provides a method for synthesizing the above-mentioned highly selective red-absorbing heptamethine cyanine dye, comprising the following steps:
[0071] S1: Accurately weigh 10mmol 2,3,3-trimethyl-3H-indole-5 sulfonic acid potassium salt and 10-10.5mmol p-Benyl bromobenzoic acid, dissolve them in 50ml acetonitrile, and protect them under a nitrogen atmosphere. Under reflux for 8 hours, cooled, rotary evaporated to remove acetonitrile, and washed with methyl tert-butyl ether or acetone several times, dried to obtain the intermediate product I.
[0072] S2: Accurately weigh 10mmol of the intermediate product I and 12mmol of diethylamine, place them in a three-necked flask containing 100ml of dichloromethane, then add 2mmol of N,N'-dicyclohexylcarboimide and 5mmol of 4 -Dimethyl...
Embodiment 2
[0091] This example provides the application of a highly selective red-absorbing heptamethine cyanine dye. The heptamethine cyanine dye synthesized in Example 1 is applied to the dark green DG0730---green camouflage coating. The dark green DG0730- --The green camouflage coating raw material composition and component mass parts are as follows:
[0092] Acrylic: 10;
[0093] The heptamethine cyanine dye: 0.25;
[0094] Flake aluminum powder: 0.5;
[0095] Pigment green: 5.0;
[0096] Pigment yellow: 4.2;
[0097] Pigment Black: 1;
[0098] Pigment red: 0.6;
[0099] Organic solvents: 6;
[0100] Other additives: 0.35 (dispersant: 0.25, defoamer: 0.1).
[0101] This embodiment also provides the above-mentioned DG0730---the preparation method of the green camouflage coating, comprising the following steps:
[0102] (1) Acrylic resin, heptamethine cyanine dye, flake aluminum powder, pigment green, pigment yellow, pigment black, pigment red, xylene, dispersant and defoamer a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full width at half maximum | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| emissivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com