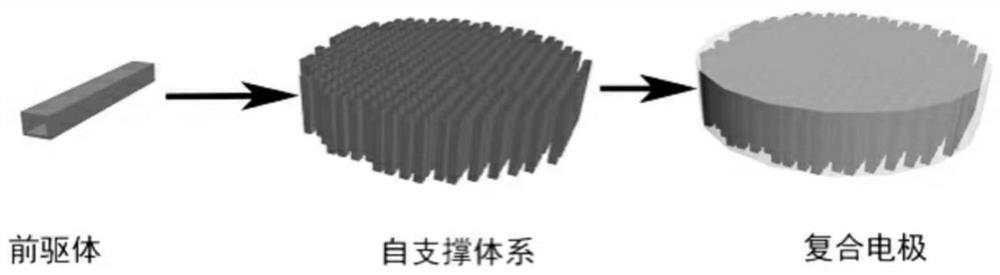
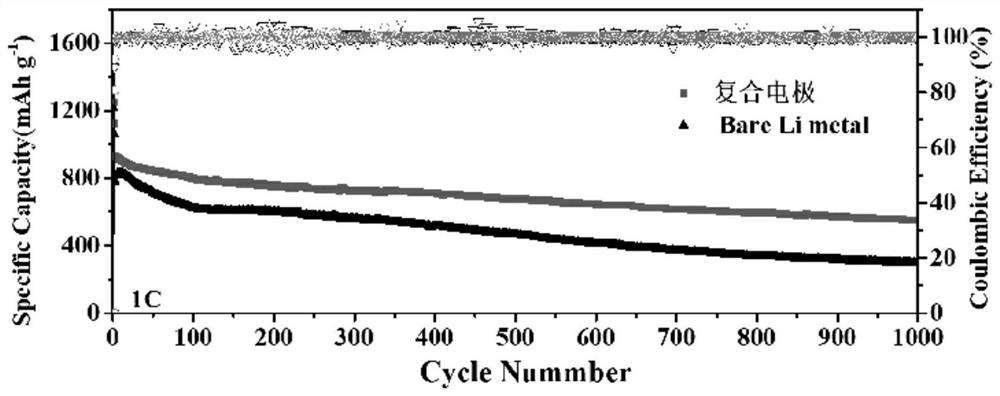
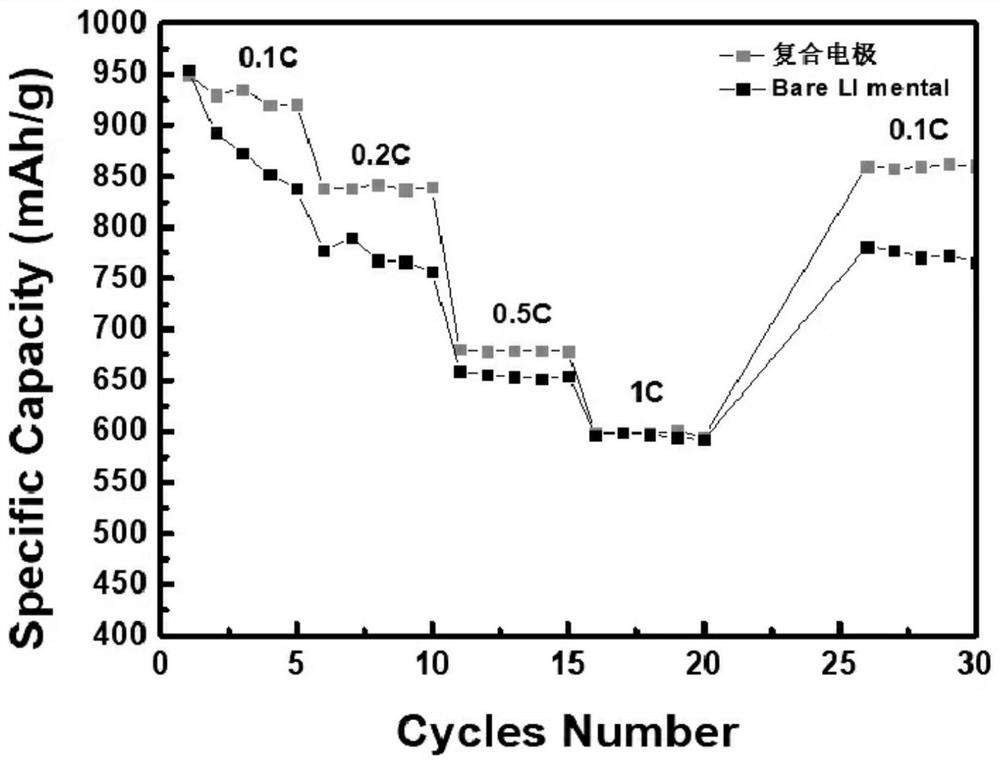
Preparation and application of a special porous composite electrode for lithium-sulfur batteries
A composite electrode and lithium-sulfur battery technology, which is applied in the direction of battery electrodes, lithium batteries, negative electrodes, etc., can solve the problems that the product cannot be applied on a large scale, the proportion of effective materials in the electrode is reduced, and the battery cycle stability is poor, so as to achieve good electronic stability. The effect of transmission ability, simple and mature preparation process, and controllable pore size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 8 mmol ascorbic acid was added to 6 mL of water and stirred for 5 minutes to obtain a clear solution A. The Cu 2 Fe(CN) 6 ·10H 2 O (0.4 mmol) was added to 74 mL of ethylene glycol and stirred for 5 minutes to give B. Then, solution A was poured into solution B for an additional 15 minutes with magnetic stirring. The prepared clear yellow solution was transferred to a 100 mL polytetrafluoroethylene-lined autoclave and kept at 70° C. for 24 hours, then naturally cooled to room temperature, and centrifuged to obtain the compound. A self-supporting copper-based membrane was obtained after vacuum filtration. The copper-based film was cut and the metal lithium was melted onto the copper-based film to make a porous composite electrode.
Embodiment 2
[0035]Add 8 mmol of vitamin C to 6 mL of water and stir for 10 minutes to obtain a clear solution A. Add Cu2Fe(CN)6 10H2O (0.4 mmol) to 74 mL of ethylene glycol and stir for 5 minutes to obtain solution B, then, magnetically stir Pour solution A into solution B for an additional 15 minutes. The prepared transparent yellow solution was transferred to a 100 mL polytetrafluoroethylene-lined autoclave and kept at 50° C. for 24 hours, then naturally cooled to room temperature, and centrifuged to obtain the compound. A self-supporting copper-based membrane was obtained after vacuum filtration. The copper-based film is cut and the metal lithium is melted onto the copper-based film to make a porous composite electrode.
Embodiment 3
[0037] Add 8 mmol of vitamin C to 6 mL of water and stir for 5 minutes to obtain a clear solution A. The Cu 2 Fe(CN) 6 (0.4 mmol) was added to 74 mL of ethylene glycol and stirred for 5 minutes to give B. Then, solution A was poured into solution B for an additional 15 minutes with magnetic stirring. The prepared transparent yellow solution was transferred to a 100 mL polytetrafluoroethylene-lined autoclave and kept at 150° C. for 24 hours, then cooled to room temperature naturally, and centrifuged to obtain the compound. A self-supporting copper-based film was obtained after mechanical pressing. The copper-based membrane was cut out and the copper-based membrane was extruded onto the lithium sheet in a glove box.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com