Preparation method of carbendazim
A technology of carbendazim and synthesis method, which is applied in the field of organic chemical synthesis of pesticide molecules, can solve the problems of high safety risk of carbendazim synthesis, achieve significant commercial value and potential, mild reaction conditions, and low reaction energy barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0017] According to one embodiment of the present invention, the synthesis method comprises the following steps:
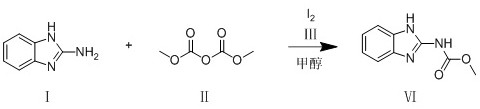
[0018] S1.2-Aminobenzimidazole is acylated with dimethyl dialkanoate in an organic solvent under the action of a catalytic reagent to generate methyl-1H-benzimidazol-2-yl-carbamate, Carbendazim;
[0019] Preferably, in step S1, the catalytic reagent is iodine, and the organic solvent is methanol.
[0020] Catalytic reagents and organic solvents are used in step S1: the catalytic reagents are iodine, triethylamine, N,N-diisopropylethylamine, 4-dimethylaminopyridine, pyridine, sodium methoxide, potassium ethoxide, tert-butanol Potassium; the organic solvent is at least one of water, dioxane, dichloromethane, tetrahydrofuran, N,N-dimethylformamide, methanol, ethanol, isopropanol or a combination thereof; preferably, the The catalytic reagent is iodine, and the organic solvent is methanol.
[0021] Compared with the existing synthetic methods, the synthetic route p...
Embodiment 1
[0031]
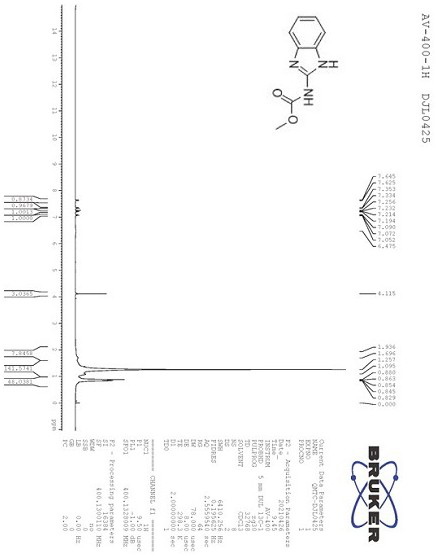
[0032] Take a round bottom flask with a built-in stirring bar. To this was added 2-aminobenzimidazole (0.2 g, 1.5 mmol), dimethyl dialkanoate (0.3 g, 2.25 mmol), iodine (38 mg, 0.15 mmol) and methanol (10 ml). The resulting mixture was stirred and reacted at room temperature for 1 hour. After the completion of the reaction was monitored by TLC, the organic solvent was evaporated to obtain a crude product. The crude product was dissolved in dichloromethane (50ml), and distilled water (50ml) was added. The organic phase was separated and the aqueous phase was washed 3 times with dichloromethane (50 ml). The organic phases were combined and evaporated to dryness, and the obtained crude product was recrystallized and purified using ethanol to obtain methyl-1H-benzimidazol-2-yl-carbamate as a white solid with a yield of 92%. The purity calculated by H NMR spectrum was 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com