Supramolecular photothermal agent compound with stimuli responsiveness and composition and application of supramolecular photothermal agent compound
A stimuli-responsive, compound technology, used in drug combinations, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as damage, reduce treatment efficiency, damage, etc., and achieve broad economic prospects and high photothermal conversion efficiency. , easily degradable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
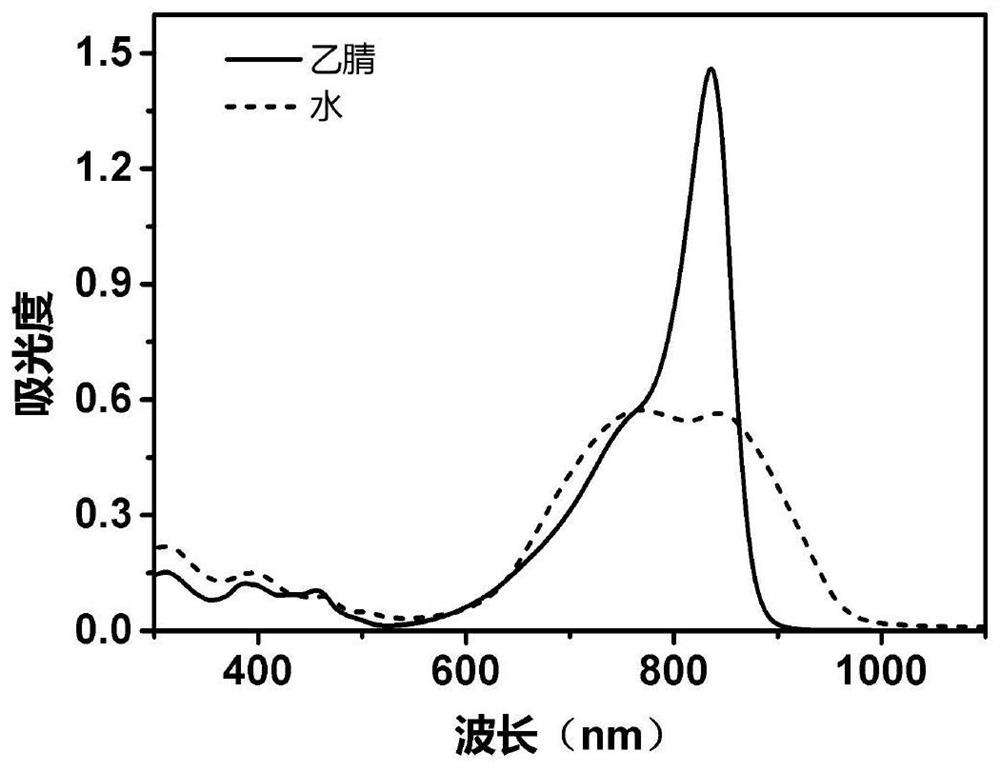
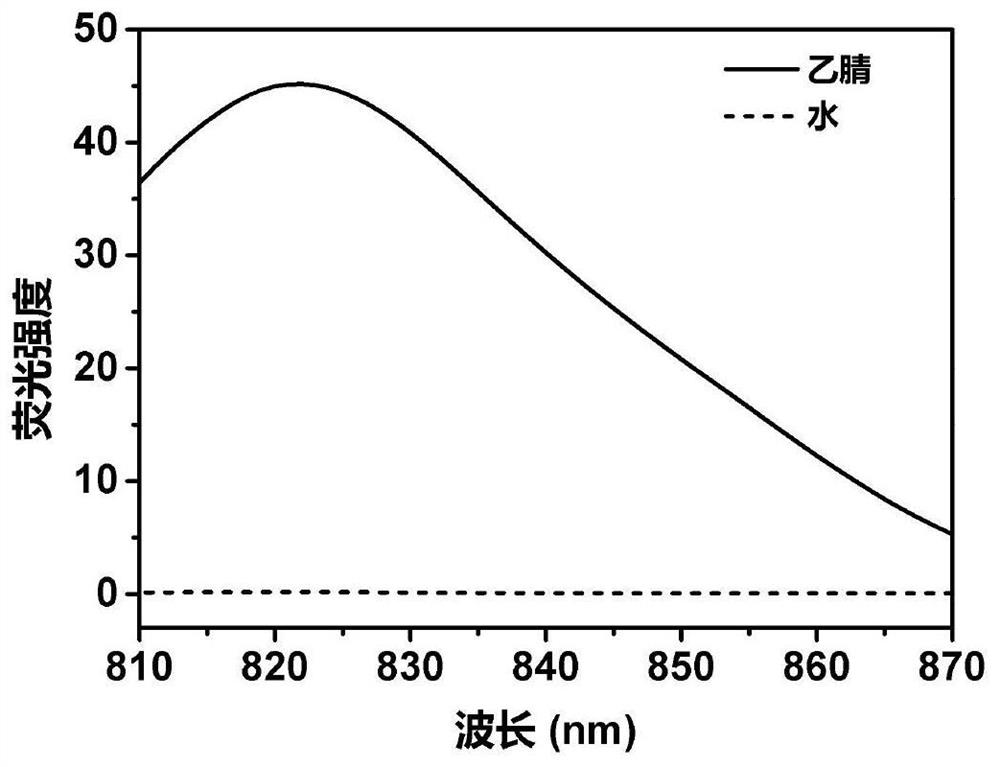
[0122] Example 1 Synthesis of compound Ⅱ-1 and its fluorescence properties
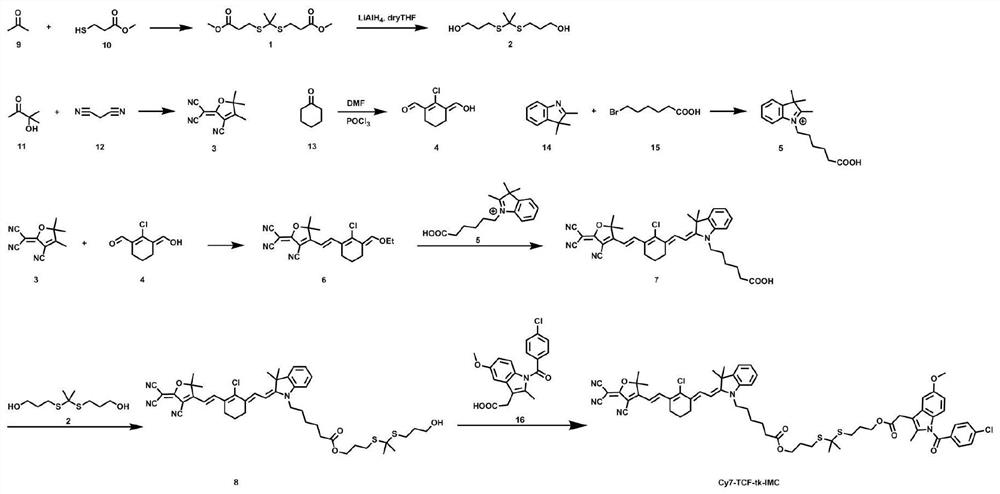
[0123] Such as figure 1 As shown, the synthesis of compound II-1 includes the following steps:
[0124] 1) Synthesis of compound 1:
[0125] 5 mL of trifluoroacetic acid (TFA) was added to the mixture of 3-mercaptopropionate and acetone, stirred at room temperature for 12 hours, and quenched by placing the mixture in an ice bath to obtain compound 1 as a colorless oil.
[0126] 1 HNMR (400MHz, CDCl 3 ): δ(ppm): 3.70(s,6H), 2.87(t,4H), 2.63(t,4H), 1.60(s,6H).
[0127] 2) Synthesis of compound 2:
[0128] Slowly add 5mL of anhydrous tetrahydrofuran (THF) to the reaction flask to dissolve LⅠAlH 4 500 mg of the oily compound 1 was slowly added dropwise under ice bath conditions, the ice bath was removed and the reaction was stirred at room temperature for 6 hours. After the reaction was completed, the reaction system was first placed in an ice-water mixture at 0°C, and then a small amount of water wa...
Embodiment 2
[0150] The synthesis of embodiment 2 compound Ⅱ-2 to Ⅱ-15
[0151] Compounds II-2 to II-15 can be prepared in a similar manner to Example 1.
[0152] 1. Synthesis of Compound Ⅱ-2
[0153]
[0154] Compound 17 was used to replace compound 4 in Example 1, and the remaining reagents and preparation methods were the same as Steps 1-9 in Example 1) to prepare Compound II-2;
[0155] 1 H NMR (400MHz, CDCl 3 )δ:7.79(d,2H),7.68(d,2H),7.40(d,1H),7.34(d,1H),7.22(t,2H),7.11(d,1H),7.06(d,1H ),6.84(d,2H),6.72(q,1H),6.70(t,1H),6.64(t,1H),6.51(m,2H),6.36(s,1H),6.23(s,1H) ,5.64(s,1H),4.71(m,1H),4.37(t,1H),4.13(m,4H),3.81(t,3H),3.77(m,2H),3.59(m,2H), 2.85(t,1H),2.42(d,4H),2.36(d,2H),2.32(d,2H),2.26(s,3H),2.11(m,2H),1.94(m,4H),1.79 (s,6H),1.59(s,6H),1.58(s,2H),1.49(m,2H),1.33(m,2H),1.16(s,6H).
[0156] 2. Synthesis of Compound Ⅱ-3
[0157]
[0158] Compound 19 was used to replace compound 5 in Example 1, and the remaining reagents and preparation methods were the same as Steps 1-9...
Embodiment 3
[0208] The preparation method of embodiment 3 micro-nano structure
[0209] Taking the self-assembled micro-nano structure of compound Ⅱ-1 as an example, dissolve Ⅱ-1 in DMSO (or organic solvents such as ethanol) to make a 2mM storage solution, and add a small amount of the storage solution to deionized water to make it 20μM working solution; take 10 μL dropwise onto a silicon wafer, observe and take pictures under a transmission electron microscope (TEM) and an atomic force microscope (AFM), the micro-nano structure in the form of vesicles can be clearly observed, and the attached Figure 4 That is the result of transmission electron microscope photographing, Figure 5 That is the particle size test distribution of DLS. From the observation results, it is found that the particle size of the micro-nano structure self-assembled by compound II-1 is about 30-150 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com