Swine streptococcosis vaccine
A technology for Streptococcus suis and vaccines, applied in vaccines, veterinary vaccines, antibacterial drugs, etc., can solve the problems of food safety, drug resistance, limited immune protection, etc., so as to reduce production costs and reduce vaccination workload. , reduce the effect of stress response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
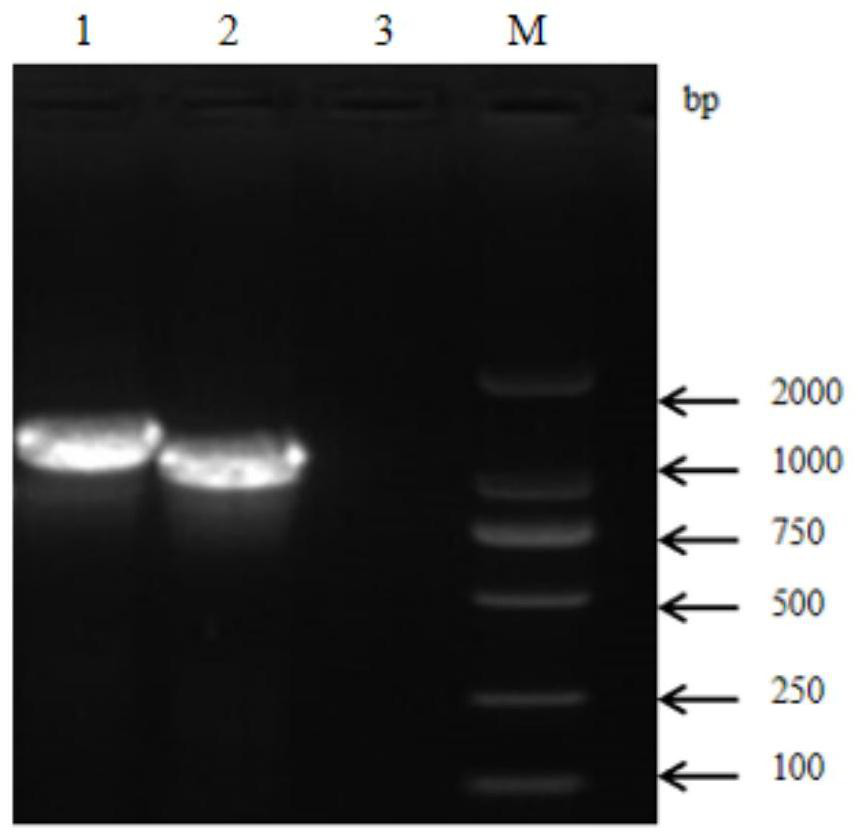
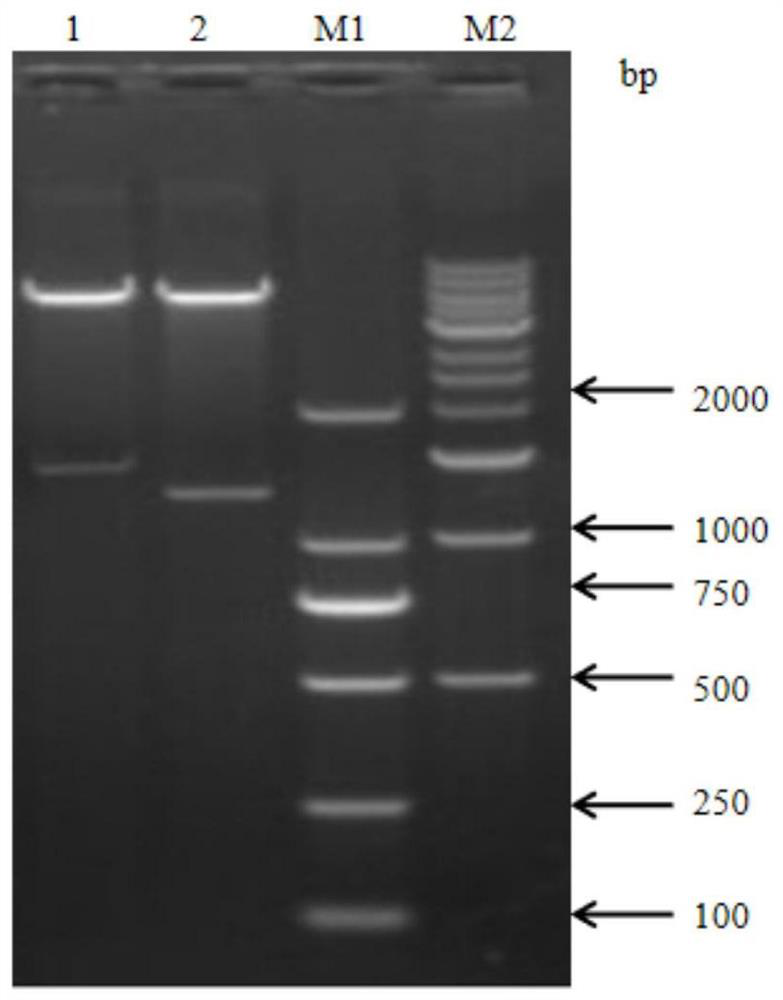
[0037] Embodiment 1 Prepares the construction of the recombinant bacterium of vaccine antigen
[0038] In order to find an effective universal vaccine antigen for Streptococcus suis, a large number of antigens were screened by immunoproteomics methods, and finally it was found that the vaccine prepared with protein A as the immunogen can protect Streptococcus suis types 2, 3 and 31 Type challenge, the gene sequence of protein A is shown in SEQ ID NO:1, and the amino acid sequence is shown in SEQ ID NO:2.
[0039] 1.PCR primers
[0040] Primers A-P1 and A-P2 were designed for amplifying the gene encoding protein A. In addition, primers B-P1 and B-P2 were designed to amplify the gene encoding the control protein, the gene sequence of the control protein is shown in SEQ ID NO:3, and the amino acid sequence is shown in SEQ ID NO:4. The specific sequences of the primers are shown in Table 1, and the primers were synthesized by Nanjing GenScript Biotechnology Co., Ltd.
[0041] T...
Embodiment 2
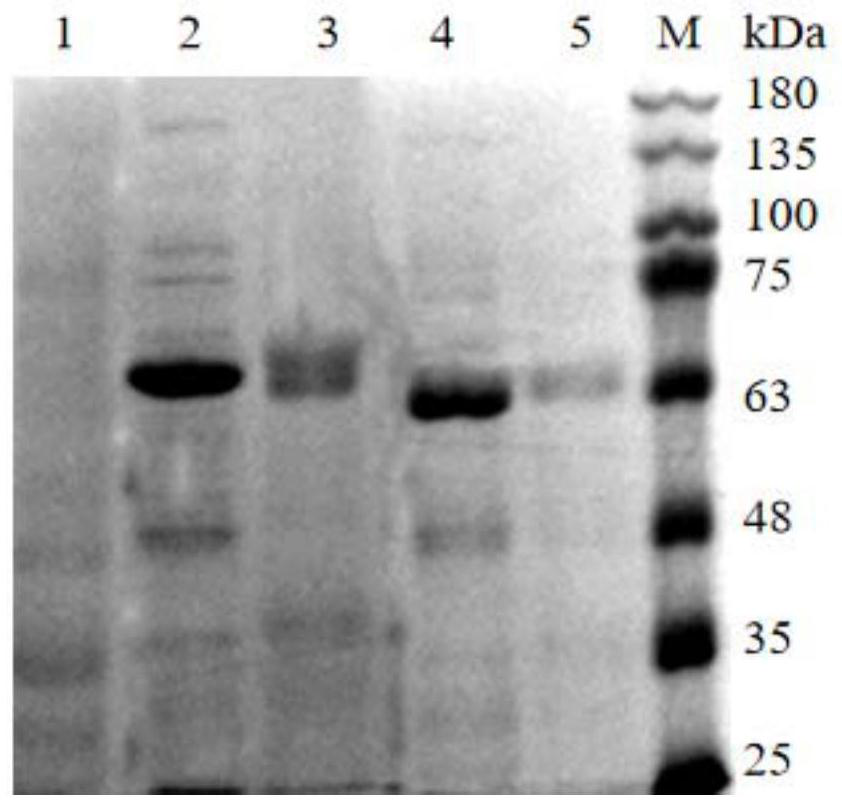
[0089] The immune effect of embodiment 2 protein A
[0090] 1. Active immunization of mice
[0091] The purified protein A was prepared into a vaccine by the following method: white oil 117.5mL, aluminum stearate 2.5g, mixed vigorously, shaken, autoclaved, cooled to about 50°C, shaken vigorously until transparent and homogeneous, The white oil adjuvant is obtained; take 1.08 mg / mL purified protein A aqueous solution, add protein A aqueous solution with 4% volume of sterilized Tween-80, and mix well to obtain the antigen solution. The antigen solution and the white oil adjuvant are mixed according to the volume ratio of 1:2, and emulsified to obtain the protein A vaccine.
[0092] Using the same method as above, the corresponding vaccine was prepared by replacing protein A with the control protein, which was designated as the control protein vaccine. The control protein vaccine and the protein A vaccine had the same antigen concentration.
[0093] According to the preparation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com