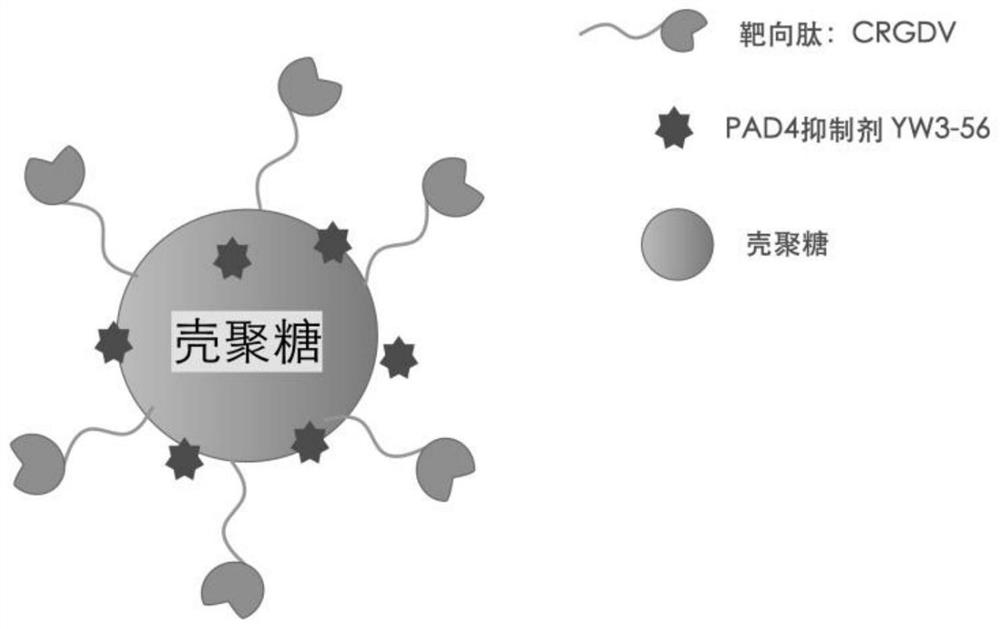
CRGD sequence peptide modified chitosan loaded PAD4 inhibitor and preparation method and application thereof
A chitosan and inhibitor technology, applied in the field of PAD4 inhibitor and its preparation, can solve the problem of low active targeting and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The present invention provides the preparation method of the PAD4 inhibitor of above-mentioned CRGD sequence peptide modification chitosan load, comprises the following steps:
[0066] (1) mixing chitosan, acryloyl chloride, the first acid-binding agent with the first organic solvent, and performing a nucleophilic substitution reaction to obtain chitosan modified by acryloyl chloride;
[0067] (2) Mix the chitosan modified by the acryloyl chloride, the CRGD sequence peptide, the second acid-binding agent with the second organic solvent, and carry out Michael addition reaction to obtain the CRGD sequence peptide modified chitosan carrier;
[0068] (3) stirring and mixing the CRGD sequence peptide modified chitosan carrier, the PAD4 inhibitor and a third organic solvent to obtain the PAD4 inhibitor loaded on the CRGD sequence peptide modified chitosan.
[0069] The invention mixes chitosan, acryloyl chloride, a first acid-binding agent and a first organic solvent to carry...
Embodiment 1
[0107] (1) Synthesis of CRGD sequence peptide
[0108] (1) Solid phase synthesis of CRGDV
[0109] 1) Swelling: Soak 300 mg of Fmoc-Val-Wang resin with 10 mL of anhydrous DMF in a solid-phase synthesis tube for 3 hours to make it swell.
[0110] 2) Deprotection: remove anhydrous DMF by suction filtration under reduced pressure, and add 8-10 mL of deprotection agent (anhydrous DMF:hexahydropyridine=4:1), shake the reaction for 3 minutes, remove the deprotection agent by suction filtration under reduced pressure, Then add 8-10mL of deprotection agent, shake and react for 8min.
[0111] 3) Washing: remove the deprotection agent by suction filtration under reduced pressure, and wash with anhydrous DMF, CH 2 Cl 2 1. Washing with anhydrous DMF, each solution was washed twice.
[0112] 4) Color development: ninhydrin method for detection of resin (detection-NH 2 ), showing dark purple.
[0113] 5) Coupling: Weigh Fmoc-Asp (OtBu) and condensing agent HBTU, dissolve with coupling...
Embodiment 2
[0150] The synthesis of embodiment 2 CRGDV-chitosan-4B
[0151] (1) Synthesis of chitosan-acryloyl chloride
[0152] First, chitosan (0.6 g, 0.20 mmol) and triethylamine (Et3N, 50 μL, 0.36 mmol) were dissolved in 20 mL of N,N-dimethylformamide (DMF). After cooling at 0°C, acryloyl chloride (8 μL, 0.10 mmol) in 8 mL of dichloromethane was added dropwise to the stirred solution. The reaction was carried out at room temperature for 24 hours, and then dialyzed against distilled water (molecular weight cut-off 700-1000Da) for 48 hours. A light yellow solid was obtained after lyophilization.
[0153] (2) Synthesis of chitosan-CRGDV
[0154] Chitosan-acryloyl chloride (0.3 g, 0.10 mmol) and CRGDV (98 mg, 0.18 mmol) were dissolved in 5 mL of dimethyl sulfoxide, and Et3N (10 μL, 0.07 mmol) was added at room temperature. The reaction was stirred at room temperature for 12-24 hours and dialyzed against distilled water (MWCO: 700-1000 Da) for 48 hours. After lyophilization, a pale ye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com