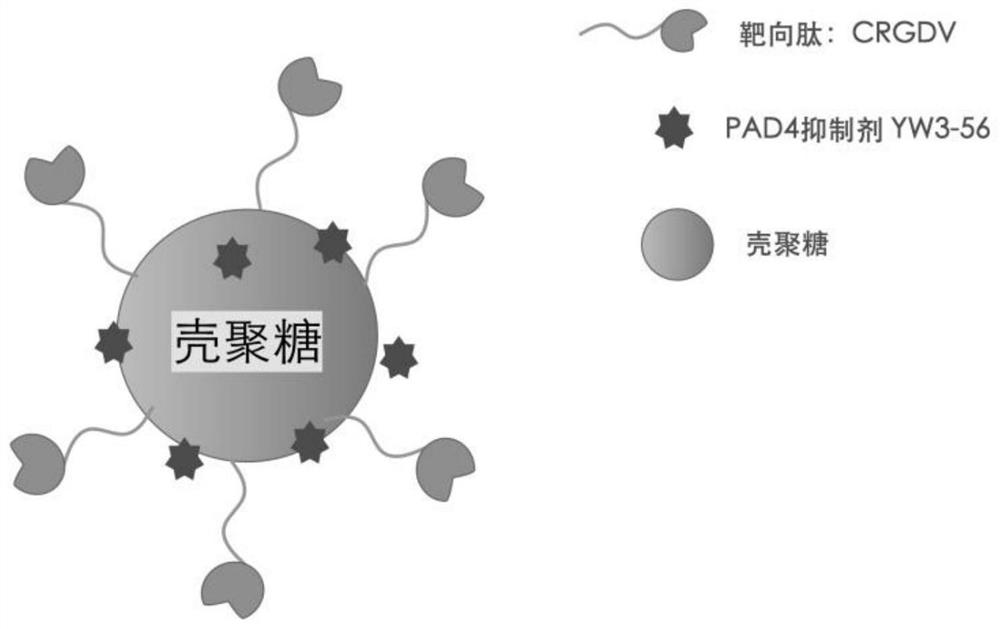
A kind of crgd sequence peptide modified chitosan-loaded pad4 inhibitor and its preparation method and application
A chitosan and inhibitor technology, applied in the field of PAD4 inhibitor and its preparation, can solve the problem of low active targeting, and achieve the effects of reducing adverse drug reactions, enhancing selectivity and good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The present invention provides a preparation method of the above-mentioned CRGD sequence peptide-modified chitosan-loaded PAD4 inhibitor, comprising the following steps:
[0066] (1) mixing chitosan, acryloyl chloride, the first acid binding agent with the first organic solvent, and carrying out a nucleophilic substitution reaction to obtain chitosan modified with acryloyl chloride;
[0067] (2) mixing the acryloyl chloride modified chitosan, the CRGD sequence peptide, the second acid binding agent and the second organic solvent, and performing a Michael addition reaction to obtain a CRGD sequence peptide modified chitosan carrier;
[0068] (3) stirring and mixing the CRGD sequence peptide-modified chitosan carrier, the PAD4 inhibitor and the third organic solvent to obtain the CRGD sequence peptide-modified chitosan-loaded PAD4 inhibitor.
[0069] In the present invention, chitosan, acryloyl chloride, a first acid binding agent and a first organic solvent are mixed to ...
Embodiment 1
[0107] (1) Synthesis of CRGD sequence peptide
[0108] (1) Solid-phase synthesis of CRGDV
[0109] 1) Swelling: In a solid-phase synthesis tube, soak 300 mg of Fmoc-Val-Wang resin with 10 mL of anhydrous DMF for 3 h to swell it.
[0110] 2) Deprotection: the anhydrous DMF was removed by suction filtration under reduced pressure, and 8-10 mL of a deprotecting agent (anhydrous DMF:hexahydropyridine=4:1) was added, and after the shaking reaction for 3 min, the deprotecting agent was removed by suction filtration under reduced pressure, Then 8-10 mL of deprotecting agent was added, and the reaction was shaken for 8 min.
[0111] 3) Washing: the deprotecting agent was removed by suction filtration under reduced pressure, followed by anhydrous DMF, CH 2 Cl 2 , washed with anhydrous DMF, and each solution was washed twice.
[0112] 4) Color development: Ninhydrin method detects resin (detection-NH 2 ), showing a deep purple color.
[0113] 5) Coupling: Weigh Fmoc-Asp (OtBu) and...
Embodiment 2
[0150] Example 2 Synthesis of CRGDV-chitosan-4B
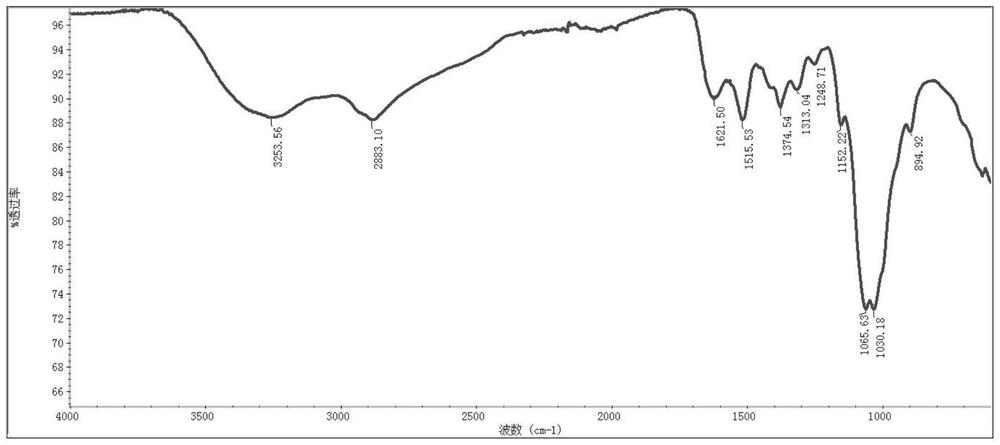
[0151] (1) Synthesis of Chitosan-Acryloyl Chloride
[0152] First, chitosan (0.6 g, 0.20 mmol) and triethylamine (Et3N, 50 µL, 0.36 mmol) were dissolved in 20 mL of N,N-dimethylformamide (DMF). After cooling at 0°C, acryloyl chloride (8 μL, 0.10 mmol) in 8 mL of dichloromethane solution was added dropwise to the stirred solution. The reaction was carried out at room temperature for 24 hours and then dialyzed against distilled water (molecular weight cut-off 700-1000 Da) for 48 hours. A pale yellow solid was obtained after lyophilization.
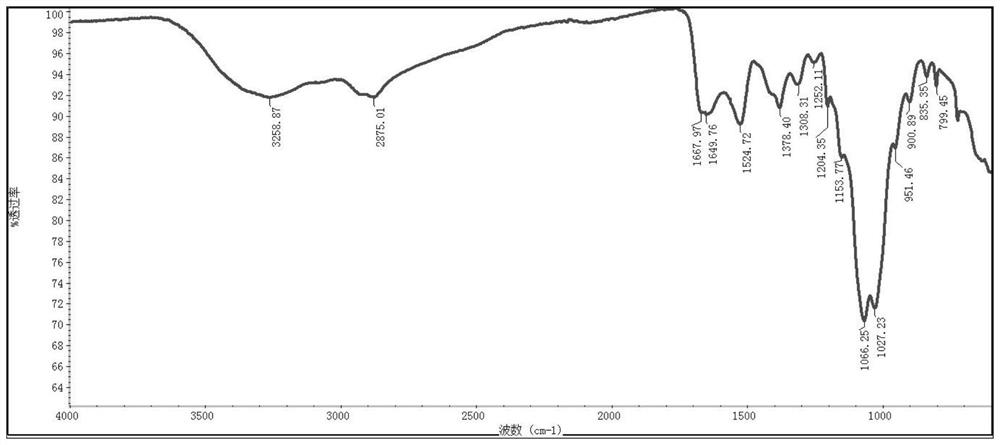
[0153] (2) Synthesis of Chitosan-CRGDV
[0154] Chitosan-acryloyl chloride (0.3 g, 0.10 mmol) and CRGDV (98 mg, 0.18 mmol) were dissolved in 5 mL of dimethyl sulfoxide and Et3N (10 μL, 0.07 mmol) was added at room temperature. The reaction was stirred at room temperature for 12-24 hours and dialyzed against distilled water (MWCO: 700-1000 Da) for 48 hours. After lyophilization, a pale ye...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com