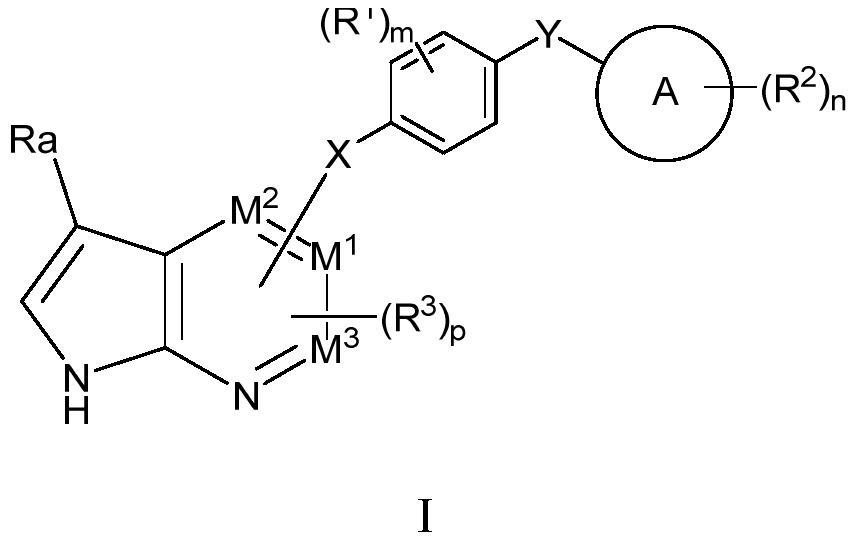
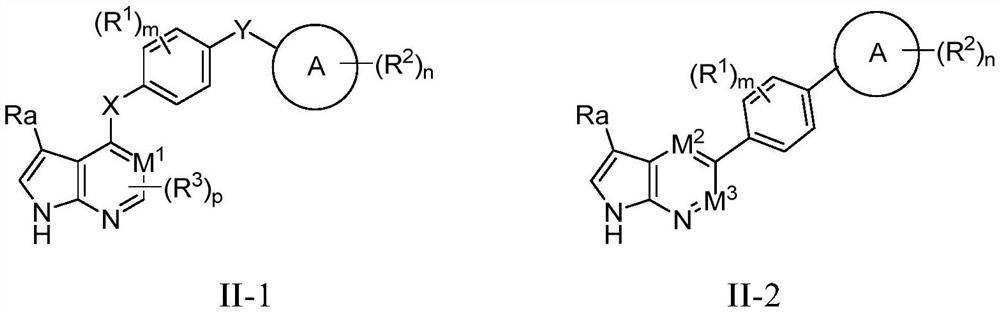
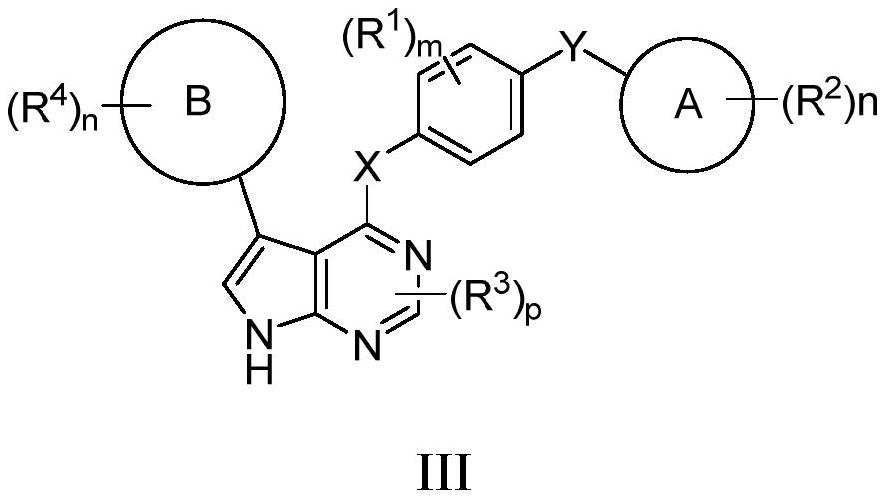
Novel HPK1 inhibitor as well as preparation method and application thereof
A C1-C6, selected technology, applied in the field of small molecule drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The preparation of formula I compound
[0089] Compounds of formula I of the present invention can be prepared by the following exemplary methods:
[0090] method 1:
[0091]
[0092] Substitution and coupling reactions are carried out with five- and six-membered compounds, and the compound of formula I is obtained after modification by substituents.
[0093] Among them, a class of compounds where A is an unsaturated ring can be prepared by method 2:
[0094]
[0095] A cyclization reaction with a compound of Ia affords a compound of formula I'.
[0096] Pharmaceutical compositions and methods of administration
[0097] Since the compound of the present invention has excellent HPK1 kinase inhibitory activity, the compound of the present invention and its various crystal forms, pharmaceutically acceptable inorganic or organic salts, hydrates or solvates, and compounds containing the compound of the present invention as the main active ingredient The pharmaceuti...
Embodiment 1
[0148] Example 1: (2-((3,5-difluoro-4-((5-(2-fluoro-4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidine-4- Base) oxo) phenyl) amino) -5-methyl-5,6-dihydro-4H-1,3-oxazin-5-yl)methanol
[0149] 3,5-Difluoro-4-((5-(2-fluoro-4-methoxyphenyl)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H- pyrrolo [2,3-d]pyrimidin-4-yl)oxo)aniline
[0150]
[0151] Under argon protection, 3,5-difluoro-4-((5-iodo-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2 ,3-d]pyrimidin-4-yl)oxo)aniline (A1, 400 mg, 0.945 mmol), (2-fluoro-4-methoxyphenyl)boronic acid (178 mg, 1.04 mmol), potassium phosphate (401 mg, 1.89 mmol) and a mixture of 1,4-dioxane (6.0 mL) / water (2.0 mL) was added [1,1'-bis(diphenylphosphino)ferrocene]dichloro Palladium chloride dichloromethane complex (76 mg, 0.094 mmol). The reaction solution was heated to 100° C. and stirred for 2 hours. The reaction was checked by LCMS. After the reaction solution was cooled to room temperature, ethyl acetate (10 mL×2) was added for extraction...
Embodiment 2
[0159] Example 2: 2-((3,5-difluoro-4-((5-(2-fluoro-4-methoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidine-4- Base) oxo) phenyl) amino) -5-fluoro-5,6-dihydro-4H-1,3-oxazin-5-yl)methanol
[0160]
[0161] 1-(3,5-difluoro-4-((5-(2-fluoro-4-methoxyphenyl)-7-((2-(trimethylsilyl)ethoxy)methyl Base)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)oxo)phenyl)-3-((3-fluorooxetan-3-yl)methyl)urea
[0162]
[0163] To a 50 mL round bottom flask equipped with a magnetic stirrer was added 3,5-difluoro-4-((5-(2-fluoro-4-methoxyphenyl)-7-((2-(trimethyl Silyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl)oxo)aniline (100 mg, 0.193 mmol) and triethylamine (79 mg, 0.77 mmol) in anhydrous dichloromethane (3.0 mL), replaced with argon three times, and then cooled the reaction solution in an ice bath. Then triphosgene (40 mg, 0.135 mmol) was added to the reaction solution, and the reaction solution was stirred at 0°C for 20 minutes. Then (3-fluorooxetan-3-yl)methanamine (102 mg, 0.969 mmol) was added to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com