Preparation method of phloroglucinol
A technology of phloroglucinol and resorcinol, applied in the field of medicine, can solve the problems of polluted environment, complicated process, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
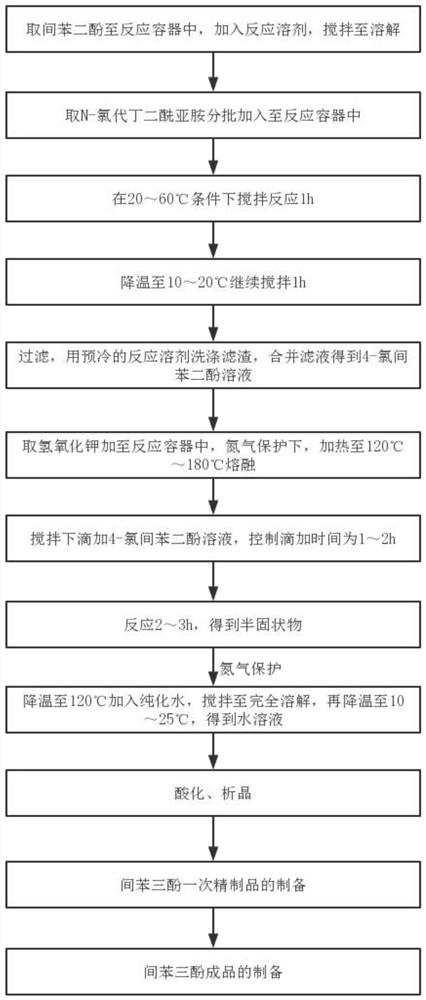
[0041] Such as figure 1 Shown, a kind of preparation method of phloroglucinol comprises the steps:
[0042] (1) Get resorcinol in reaction container, add reaction solvent, and reaction solvent comprises any one in chloroform, dichloromethane, acetone, butanone, methyl tert-butyl ether or ether. Stir until dissolved; take N-chlorosuccinimide and add it to the reaction vessel in batches, the molar ratio of N-chlorosuccinimide to resorcinol is 0.8-1.5:1, control the temperature Over 60°C; after the addition, stir and react at 20-60°C for 1 hour, preferably at a reaction temperature of 30-60°C. Then lower the temperature to 10-20° C. and continue stirring for 1 h, filter, wash the filter residue with a pre-cooled reaction solvent, combine the filtrates to obtain a 4-chlororesorcinol solution, and take it out for later use.
[0043] (2) Add potassium hydroxide to the reaction vessel, the molar ratio of potassium hydroxide to 4-chlororesorcinol is 6-20:1. Under the protection of ...
Embodiment 1
[0048] The present embodiment provides a kind of preparation method of phloroglucinol, comprising the following steps:
[0049] (1) Take 110.0g of resorcinol into the reaction vessel, add 440ml of ether, and stir until completely dissolved.
[0050] Take N-chlorosuccinimide and add it to the reaction vessel in 10 batches. The molar ratio of N-chlorosuccinimide to resorcinol is 1.1:1, and the temperature is controlled not to exceed 40°C. After the addition, stir and reflux at 40°C for 1 hour; then lower the temperature to 10-20°C and continue stirring for 1 hour, filter, wash the filter residue with pre-cooled ether, combine the filtrates to obtain 4-chlororesorcinol solution, take it out for later use.
[0051] (2) Take potassium hydroxide into the reaction vessel, the molar ratio of potassium hydroxide to 4-chlororesorcinol is 8:1. Under the protection of nitrogen, heat the potassium hydroxide in an environment of 145° C. to melt, then add the 4-chlororesorcinol solution pre...
Embodiment 2
[0056] The present embodiment provides a kind of preparation method of phloroglucinol, comprising the following steps:
[0057] (1) Take 110.0 g of resorcinol into a reaction vessel, add 330 ml of reaction solvent methyl tert-butyl ether, and stir until completely dissolved.
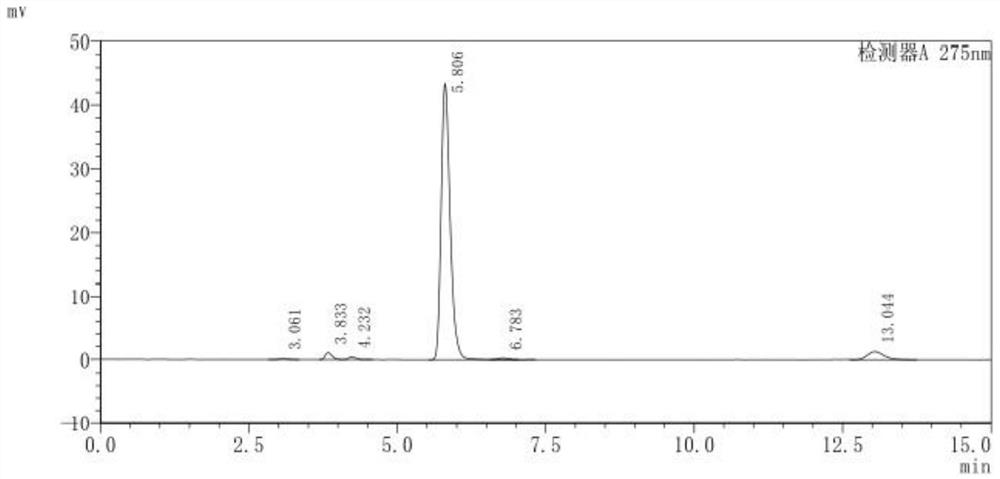
[0058] Take N-chlorosuccinimide and add it to the reaction vessel in 10 batches, the molar ratio of N-chlorosuccinimide to resorcinol is 1:1, and the temperature is controlled not to exceed 50°C. After the addition, stir and react at 45°C for 1h; then lower the temperature to 10-20°C and continue stirring for 1h, filter, wash the filter residue with pre-cooled methyl tert-butyl ether, and combine the filtrates to obtain 4-chlororesorcinol solution , 4-chlororesorcinol content determination high performance liquid chromatogram is as figure 2 shown.
[0059] (2) Take potassium hydroxide into the reaction vessel, the molar ratio of potassium hydroxide to 4-chlororesorcinol is 10:1. Under the protection ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com