Application of diterpenoid compound in preparation of beta-glucuronidase activity inhibitor
An activity inhibitor, aldolidase technology, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation and Identification of Example 1 Compound 1
[0033] 1. Preparation of Compound 1
[0034] (1) After cutting and crushing the dried Tie Haitang (3kg), leaching with 18 liters of 95% ethanol at room temperature (25-30°C) for seven days, filtered, and repeated leaching of the filter cake twice (18 liters each time, seven days each time) , combined the extracts and concentrated to dryness under reduced pressure to obtain 364.5 g of extract extract;
[0035] (2) Dissolve 364.5 g of the extract extract from the above step (1) in 1 liter of chloroform, back-extract 3 times with water (3 liters each), take the organic phase and concentrate it to dryness under reduced pressure to obtain a chloroform extract (222 g) .
[0036] (3) step (2) chloroform extract is dissolved with 200mL dichloromethane, carries out open silica gel column chromatography (silica gel 200-300 order, silica gel column diameter d=13cm, height h=60cm), successively with volume ratio as 20: 1, ...
Embodiment 2
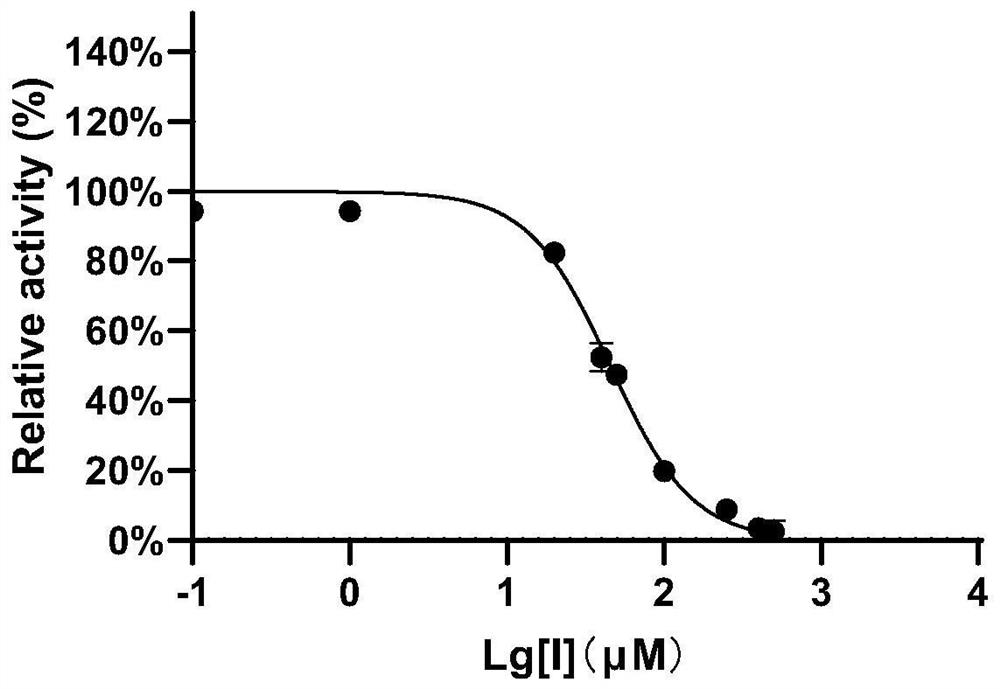
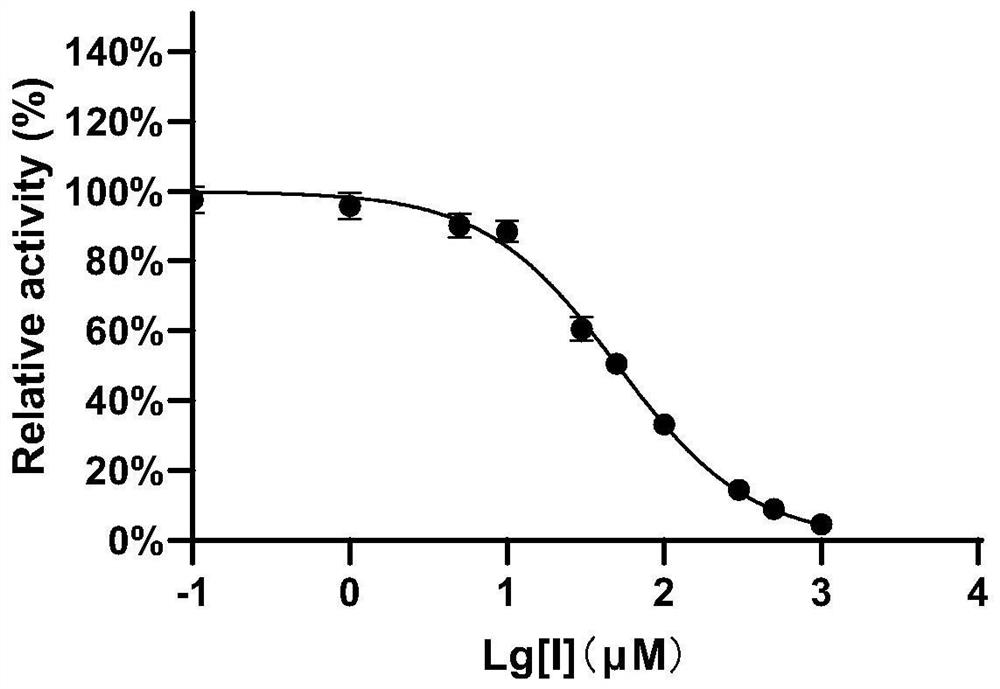
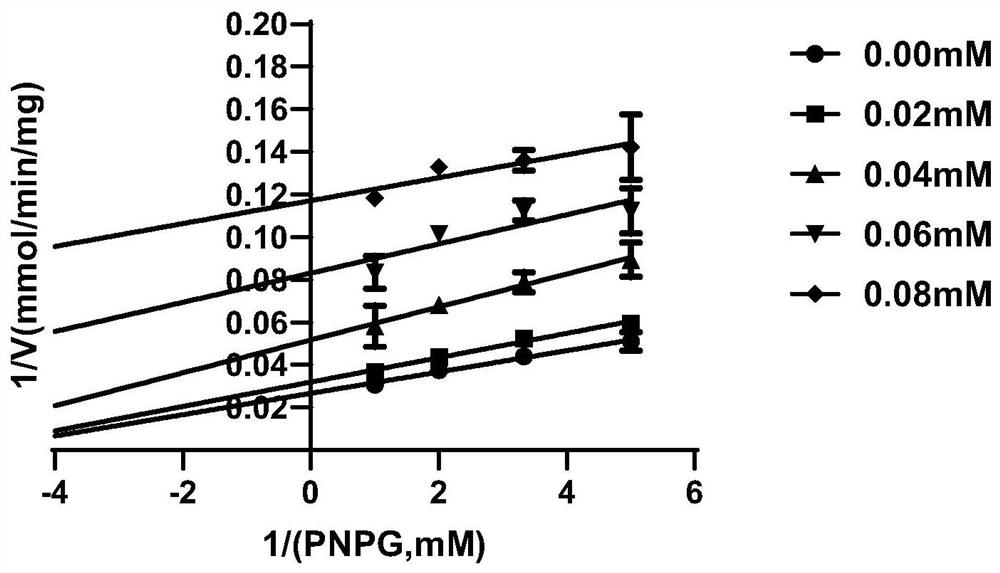
[0043] Example 2 The inhibitory effect of compound 1 on EcGUS
[0044] 1. Preparation of β-glucuronidase (EcGUS):
[0045] LB liquid medium: trypsin 10g / L, yeast extract 5g / L, sodium chloride 10g / L, solvent is water, pH7.0.
[0046] Lysis solution: 20mM 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), 300mM NaCl, 5mM imidazole, volume concentration 10% glycerol, solvent is water, pH 7.4.
[0047] NTA-0 buffer: 20 mM Tris-HCl, 0.5 M NaCl, volume solubility 10% glycerol, pH 7.9.
[0048] NTA-20 buffer: 20mM Tris-HCl, 0.5M sodium chloride, volume solubility 10% glycerol, 20mM imidazole, pH7.9.
[0049] NTA-250 buffer: 20mM Tris-HCl, 0.5M sodium chloride, volume solubility 10% glycerol, 250mM imidazole, pH7.9.
[0050] Inoculate Escherichia coli (Escherichia coli BL21 (DE3), stored at -80°C) into 200 mL of LB liquid medium containing 30 μg / mL kanamycin, and cultivate it at 200 rpm and 37°C until the OD600 reaches 0.5, then add Isopropyl-β-D-thiogalactopyranoside (IPTG) wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com