Supramolecular ionic liquid catalyst, preparation method and application thereof
An ionic liquid and catalyst technology, applied in chemical instruments and methods, preparation of organic compounds, catalysts for physical/chemical processes, etc., can solve problems such as easy unloading, reduction of ionic liquid active component concentration contact area, affecting catalytic efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A preparation method of supramolecular ionic liquid catalyst, the steps are as follows:
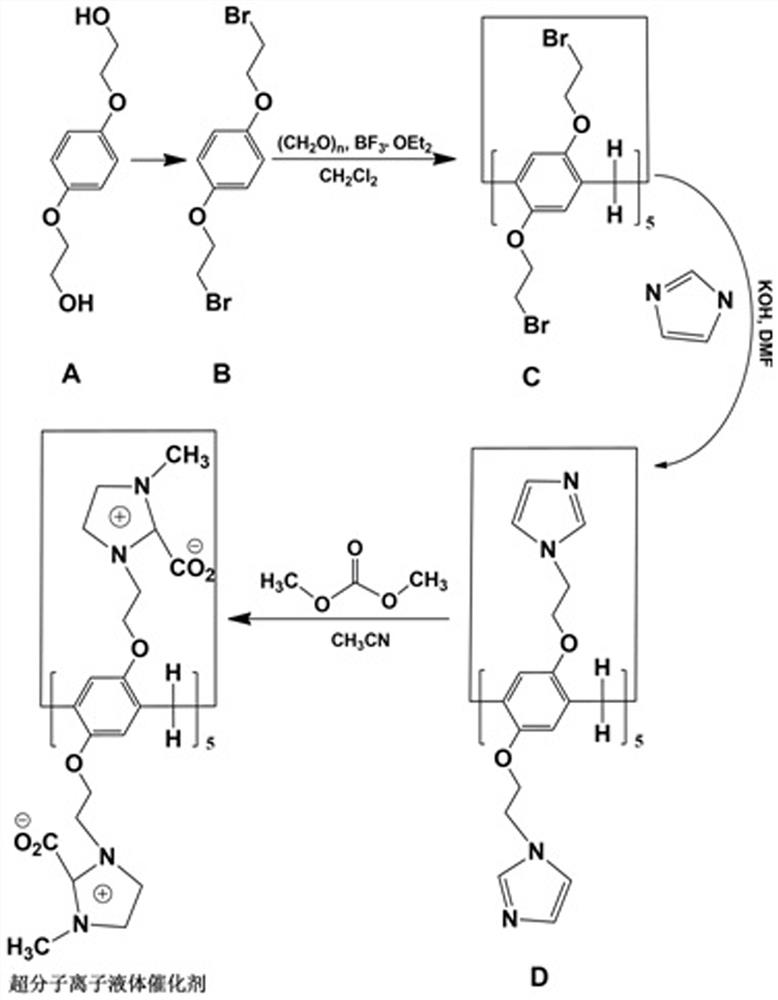
[0045] Structural design and preparation routes of supramolecular ionic liquid catalysts, such as figure 1 shown. Among them, 1,4-bis(2-hydroxyethoxy)benzene is the raw material, and B, C, and D are intermediate products.
[0046] Step 1, Synthesis of Intermediate B
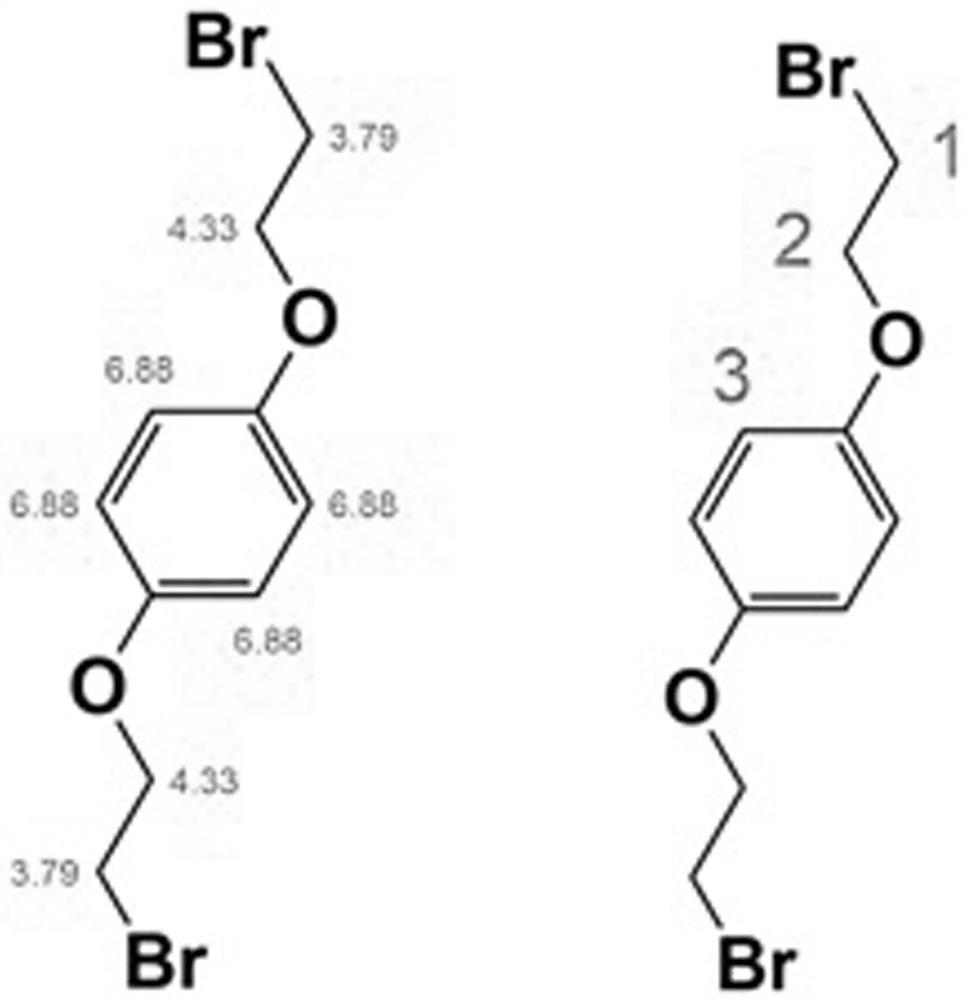
[0047] Synthesis of Intermediate B: Dissolve 1,4-bis(2-hydroxyethoxy)benzene and triphenylphosphine in anhydrous acetonitrile, 1,4-bis(2-hydroxyethoxy)benzene, triphenyl The mass ratio of phosphine and anhydrous acetonitrile is 3-5:8-10:100, cooled in an ice-water bath; under stirring, slowly add CBr 4 , CBr 4 The mass ratio with anhydrous acetonitrile is 1-2:10, and the mixture is stirred at room temperature for 12 hours; add cold water with a mass ratio of 1:1 to acetonitrile (temperature is lower than 10°C), and a white precipitate is precipitated. Suction filtration under reduced pressure, filter cake is coll...
Embodiment 1
[0063] Step 1, the synthesis of intermediate B1
[0064] Synthesis of intermediate B1: 1,4-bis(2-hydroxyethoxy)benzene (3g) and triphenylphosphine (10g) were dissolved in anhydrous acetonitrile (100g), and cooled in an ice-water bath. With stirring, slowly add CBr 4 (20g), the mixture was stirred at room temperature for 12h. Cold water (100 g) was added and a white precipitate precipitated. Suction filtration under reduced pressure, the filter cake was collected and dried to obtain white flaky solid B1.
[0065] Step 2, the synthesis of intermediate C1
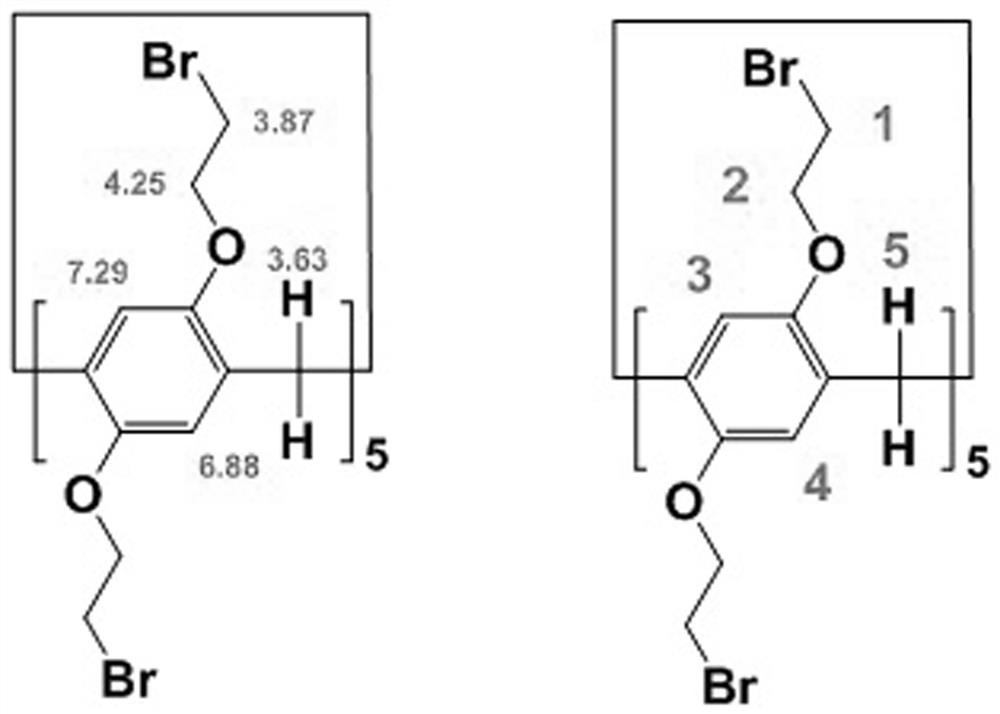
[0066] Synthesis of intermediate C1: Add intermediate B1 (3 g), paraformaldehyde (1 g), CH 2 Cl 2 (100g), the mixture was cooled in an ice-water bath for 10 min under the protection of nitrogen (purity 99%), and BF was added 3 -Et 2 0 (3g), the mixture was stirred for 2.5h. The mixture was suction filtered, and the filtrate was washed with 2.5 mol / L NaOH solution (60 mL) and distilled water (30 mL) and then washed with...
Embodiment 2
[0072] Step 1, the synthesis of intermediate B2
[0073] Synthesis of intermediate B2: 1,4-bis(2-hydroxyethoxy)benzene (5g) and triphenylphosphine (8g) were dissolved in anhydrous acetonitrile (100g), and cooled in an ice-water bath. With stirring, slowly add CBr 4 (10g), the mixture was stirred at room temperature for 12h. Cold water (100 g) was added and a white precipitate precipitated. Suction filtration under reduced pressure, the filter cake was collected and dried to obtain white flaky solid intermediate B2.
[0074] Step 2, the synthesis of intermediate C2
[0075] Synthesis of intermediate C2: Add intermediate B2 (6 g), paraformaldehyde (2 g), CH 2 Cl 2 (100g), the mixture was cooled in an ice-water bath for 30 min under the protection of nitrogen (purity 99%), and BF was added 3-Et 2 0 (6g), the mixture was stirred for 2.5h. The mixture was filtered with suction, and the filtrate was washed with 2.5 mol / L NaOH solution (2 x 30 mL) and distilled water (1 x 30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com