Synthesis method for preparing m-aminoacetanilide hydrochloride from dinitrochlorobenzene
A technology of aminoacetanilide hydrochloride and dinitrochlorobenzene is applied in the synthesis field of dinitrochlorobenzene to prepare m-aminoacetanilide hydrochloride, and can solve problems such as low efficiency, low safety factor, and large resource consumption. , to achieve the effect of ensuring simplicity, realizing reuse, and reducing energy costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
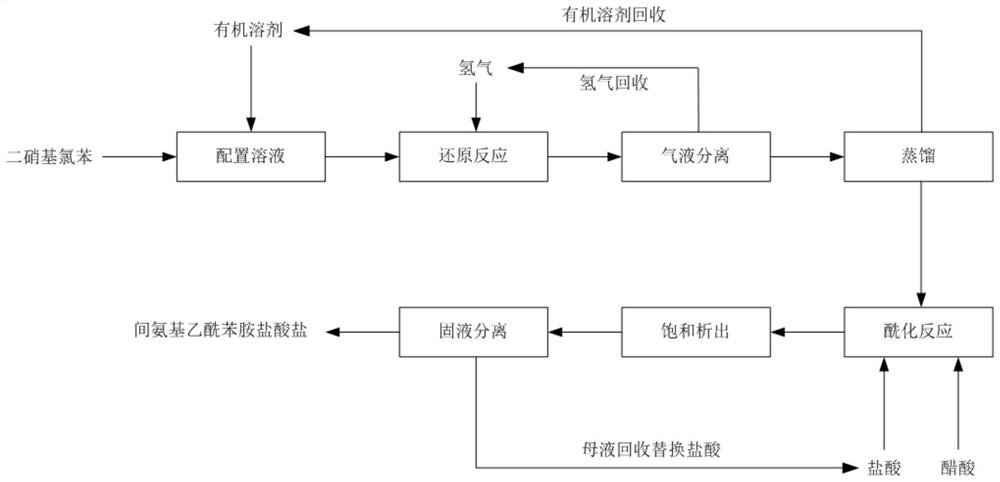
[0035] The mixture of 2,4-dinitrochlorobenzene (60wt%) and 2,6-dinitrochlorobenzene (40wt%) is dissolved in methanol with a mass ratio of 1:10 to obtain the methanol of dinitrochlorobenzene solution; the methanol solution of dinitrochlorobenzene is preheated to 90 ° C, and hydrogen is continuously input into a fixed-bed microreactor for reduction hydrogenation reaction. The fixed catalyst in the reactor is platinum carbon to control the dinitrochlorobenzene The molar ratio of hydrogen is 1:8, the reaction pressure is 2.5MPa, and the reaction time is 60s; after the reaction, gas-liquid separation and recovery of hydrogen; the reduction solution is distilled under reduced pressure to recover methanol and water, and then preheated with acetic acid and hydrochloric acid to 90 ℃, then enter the reactor for acylation reaction, control the mass ratio of dinitrochlorobenzene to acetic acid and hydrochloric acid to be 1:0.5:2.5, the concentration of hydrochloric acid is 20wt%, and the r...
Embodiment 2
[0037] Dissolve 2,4-dinitrochlorobenzene in ethanol at a mass ratio of 1:5 to obtain an ethanol solution of dinitrochlorobenzene; preheat to 145°C, and continuously input hydrogen into a fixed-bed microreactor for reduction reaction. The catalyst fixed in the reactor is nickel / silicon dioxide, the molar ratio of dinitrochlorobenzene and hydrogen is controlled to be 1:10, the reaction pressure is 2MPa, and the reaction time is 80s; The solution is distilled under reduced pressure to recover ethanol and water, preheated with acetic acid and hydrochloric acid to 85°C, and then input into the reactor for acylation reaction, controlling the mass ratio of dinitrochlorobenzene to acetic acid and hydrochloric acid to be 1:0.6:2.7, The concentration was 18wt%, and the reaction time was 11 hours. After the reaction, the temperature was lowered to 15°C to precipitate solids, and the product m-aminoacetanilide hydrochloride was separated by centrifugation. The mother liquor was recovered a...
Embodiment 3
[0039]Dissolve 2,6-dinitrochlorobenzene in methanol at a mass ratio of 1:8 to obtain a methanol solution of dinitrochlorobenzene; preheat to 40°C, and continuously input hydrogen into a fixed-bed microreactor for reduction reaction. The catalyst fixed in the reactor is ruthenium carbon, the molar ratio of dinitrochlorobenzene and hydrogen is controlled to be 1:8, the reaction pressure is 4.8MPa, and the reaction time is 25s; Recover methanol and water by distillation under reduced pressure. After preheating with acetic acid and hydrochloric acid to 80°C, they are input into the reactor for acylation reaction. 22 wt%, and the reaction time is 17 hours. After the reaction, the temperature was lowered to 20°C to precipitate a solid, and the product m-aminoacetanilide hydrochloride was separated by centrifugation. The mother liquor was recovered and replaced with hydrochloric acid for the next batch of acylation reactions. The purity of the obtained m-aminoacetanilide hydrochlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com