Sulfonium salt cationic polymer with antibacterial property, preparation method and application
A cationic polymer and salt technology, applied in the directions of botanical equipment and methods, applications, fungicides, etc., can solve the problems of acute toxicity and skin irritation, poor broad-spectrum, low antibacterial activity, etc., and achieve good industrial application prospects , the effect of simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
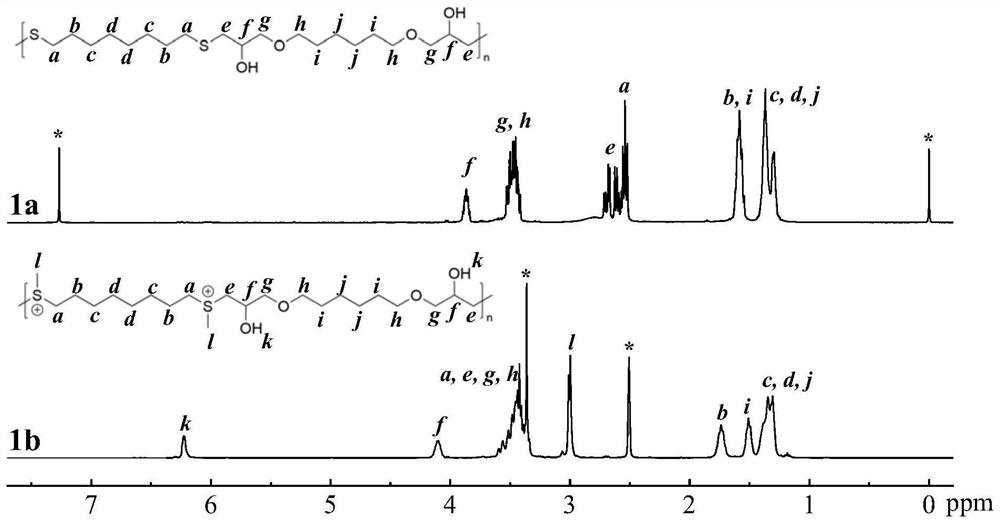
[0071] This example provides the preparation method of the sulfonium salt cationic polymer of the repeating unit shown in formula I-1, the specific operation is as follows:
[0072]
[0073] a. Weigh 1,6-hexanediol (10mmol) in a reaction flask, add epichlorohydrin (60mmol), then add tetrabutylammonium bromide (0.5mmol), sodium hydroxide (30mmol) and 0.2 mL of deionized water, the mixed solution was reacted at 40°C for 4 hours, after the reaction was completed, it was filtered, washed and dried to obtain 1,6-hexanediol diglycidyl ether;
[0074] b. Weigh 1,6-hexanediol diglycidyl ether (2.61mmol) and 1,8-octanedithiol (2.61mmol), dissolve in tetrahydrofuran / deionized water (9:1, 2.925mL) and mix solution, followed by adding triethylamine (3.91mmol), allowing it to react with stirring at room temperature for 24h to obtain an alternating copolymer containing repeating units shown in formula I-1-a;
[0075]
[0076] c. Weigh the alternating copolymer (1.58mmol) of the repea...
Embodiment 2
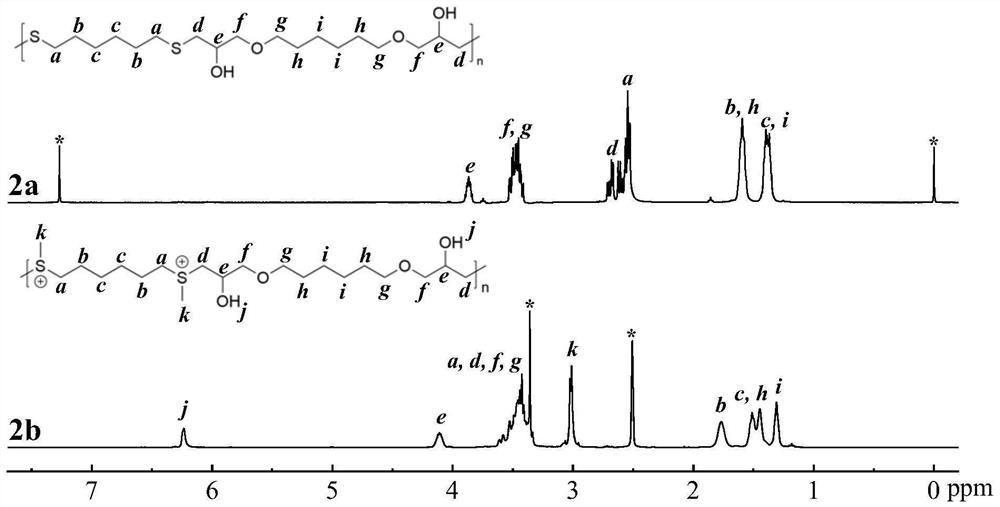
[0078] This example provides the preparation method of the sulfonium salt cationic polymer of the repeating unit shown in formula I-2, and the specific operation steps are as follows:
[0079]
[0080] a. Weigh 1,6-hexanediol (10mmol) in a reaction flask, add epichlorohydrin (60mmol), then add tetrabutylammonium bromide (0.5mmol), sodium hydroxide (30mmol) and 0.2 mL of deionized water, the mixed solution was reacted at 40°C for 4 hours, after the reaction was completed, it was filtered, washed and dried to obtain 1,6-hexanediol diglycidyl ether;
[0081] b. Weigh 1,6-hexanediol diglycidyl ether (2.61mmol) and 1,6-hexanedithiol (2.61mmol), dissolve in tetrahydrofuran / deionized water (9:1, 2.687mL) and mix solution, followed by adding triethylamine (3.91mmol), allowing it to react with stirring at room temperature for 24h to obtain an alternating copolymer containing repeating units shown in formula I-2-a;
[0082]
[0083] c. Weigh the alternating copolymer (1.582mmol) ...
Embodiment 3
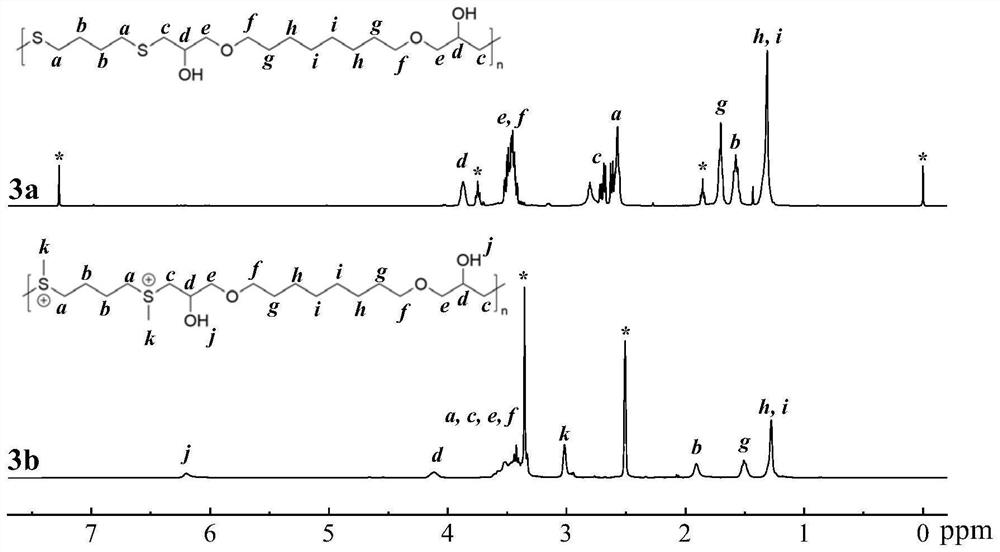
[0085] This embodiment provides the preparation method of the sulfonium salt cationic polymer of the repeating unit shown in formula I-3, the specific operation is as follows:
[0086]
[0087] a. Weigh 1,8-octanediol (10mmol) in a reaction flask, add epichlorohydrin (60mmol), then add tetrabutylammonium bromide (0.5mmol), sodium hydroxide (30mmol) and 0.2 mL of deionized water, and place the mixed solution at 40°C for 4 hours. After the reaction, filter, wash and dry to obtain 1,8-octanediol diglycidyl ether;
[0088] b. Weigh 1,8-octanediol diglycidyl ether (0.776mmol) and 1,4-butanedithiol (0.776mmol), dissolve in tetrahydrofuran / deionized water (9:1, 0.961mL) and mix solution, followed by adding triethylamine (1.163mmol), allowing it to react with stirring at room temperature for 24h to obtain an alternating copolymer containing repeating units shown in formula I-3-a;
[0089]
[0090] c. Weigh the alternating copolymer (0.350 mmol) of the repeating unit shown in fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com