Synthesis method of Pexidartinib intermediate
A synthesis method and intermediate technology, which is applied in the field of synthesis of the anticancer drug pecidatinib intermediate, can solve the problems of lack of market competitiveness in large-scale production and expensive raw materials, and achieve easy scale production promotion and low cost. Low cost, easy to achieve the effect of process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
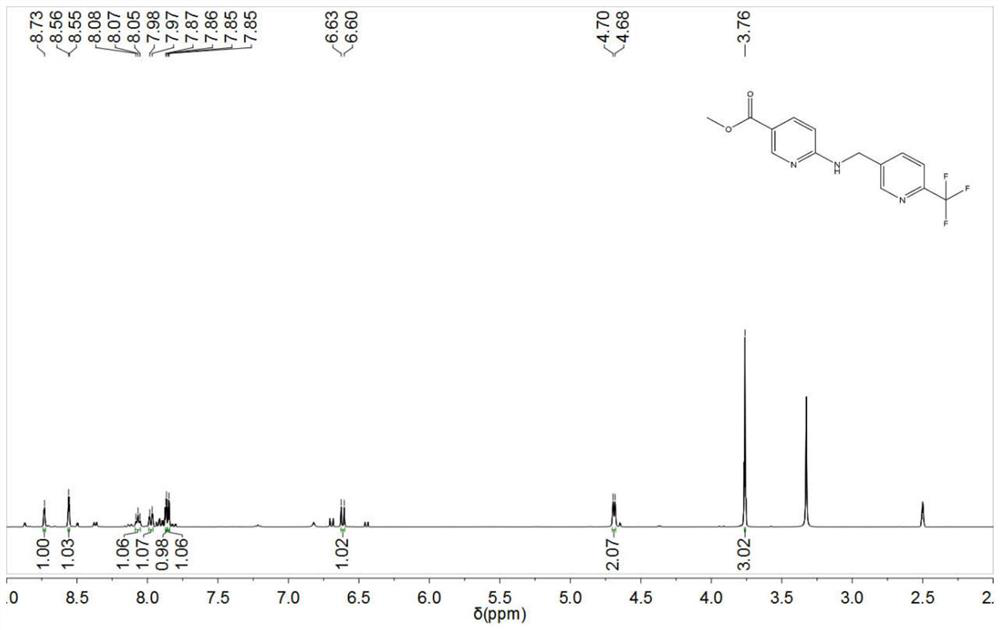
[0048] In a 100ml single-necked flask, add 10ml of acetonitrile, 86.9mg of 6-aminonicotinic acid methyl ester, stir for 10min until completely dissolved, then add 100mg of 6-trifluoromethylnicotinaldehyde, continue stirring for 20min, add sodium borohydride 108mg, glacial acetic acid 172mg, heated to 50°C, kept for 36h, then poured the reaction liquid into 50ml of 10% potassium carbonate aqueous solution, added 50ml of ethyl acetate to stir and extract, collected the organic phase after layering, concentrated and dried to obtain 168mg of yellow solidified product, Calculated yield is 94.51%, proton nuclear magnetic resonance spectrum detects as figure 1 Shown: The specific analysis results are as follows:
[0049] 1 H NMR (400MHz, dmso): δ8.73(s, 1H), 8.56(d, J=2.2Hz, 1H), 8.07(t, J=5.8Hz, 1H), 7.98(d, J=6.8Hz, 1H), 7.87(d, J=1.8Hz, 1H), 7.85(d, J=2.6Hz, 1H), 6.61(d, J=8.8Hz, 1H), 4.69(d, J=5.8Hz, 2H) ,3.76(s,3H).
[0050] It can be known that the obtained compound is 6-({...
Embodiment 2
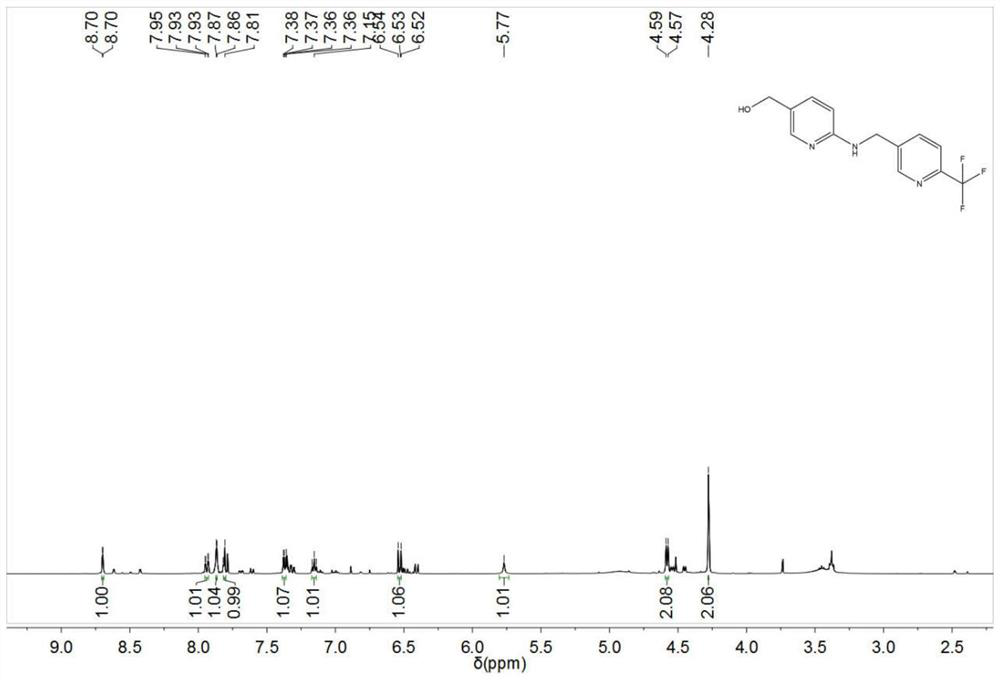
[0058] In a 100ml single-necked flask, add 10ml of acetonitrile and 78.7mg of 6-aminonicotinic acid, stir for 10min until they are completely dissolved, then add 110mg of 6-trifluoromethylnicotinaldehyde, continue stirring for 20min, then add 107mg of sodium cyanoborohydride, 10% 100 mg of ferric chloride solution, heated to 25°C, and kept for 10 hours, then poured the reaction solution into 50 ml of 10% potassium carbonate aqueous solution, added 50 ml of ethyl acetate to stir and extract, collected the organic phase after layering, concentrated and dried, and obtained yellow The solid was 157 mg, and the calculated yield was 92.68%. The yellow solid was detected as 6-({[6-(trifluoromethyl)pyridin-3-yl]methyl}amino)pyridine-3-carboxylic acid.
[0059] In a 100ml three-necked flask, add 100mg of 6-({[6-(trifluoromethyl)pyridin-3-yl]methyl}amino)pyridine-3-carboxylic acid, then add 10ml of anhydrous tetrahydrofuran, stir for 30min, and slowly drop Add 45mg of diisobutyl aluminu...
Embodiment 3
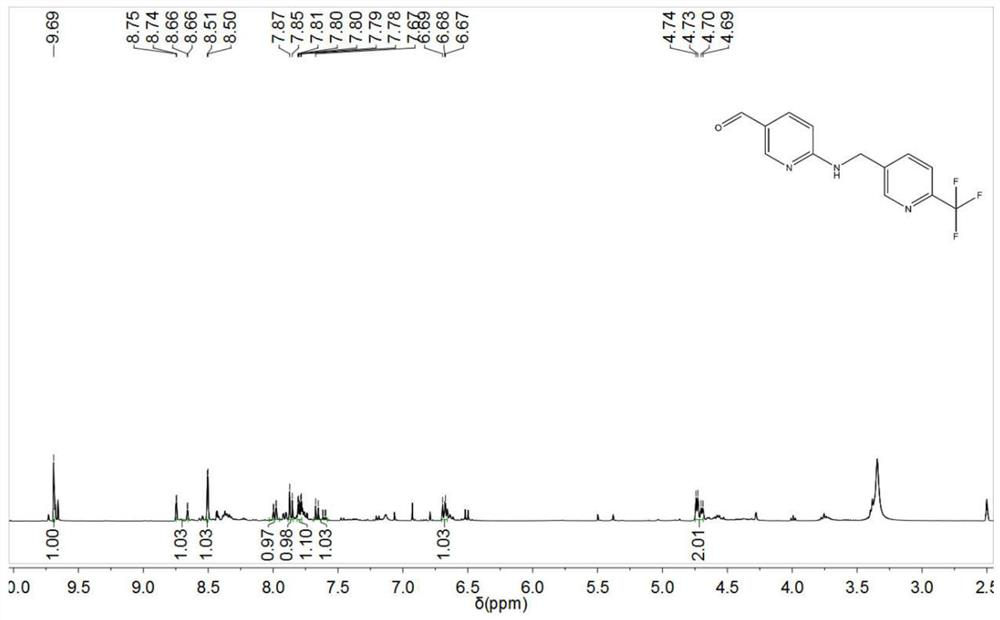
[0062] In a 100ml single-necked flask, add 10ml of acetonitrile and 94.7mg of ethyl 6-aminonicotinate, stir for 10min until they are all dissolved, then add 130mg of 6-trifluoromethylnicotinaldehyde, continue stirring for 20min, and then add triacetoxy borohydrogenate Sodium 724.7mg, hydrochloric acid-magnesium chloride mixed solution 100mg, heated to 80°C, heat preservation reaction for 48h, then pour the reaction solution into 50ml of 10% potassium carbonate aqueous solution, add 50ml of ethyl acetate, stir and extract, collect the organic phase after layering, concentrate Dried to obtain 176.5 mg of yellow solid, the calculated yield was 95.26%, and the yellow solid was detected as 6-({[6-(trifluoromethyl)pyridin-3-yl]methyl}amino)pyridine-3- Ethyl formate.
[0063] In a 100ml three-necked flask, add 100mg of ethyl 6-({[6-(trifluoromethyl)pyridin-3-yl]methyl}amino)pyridine-3-carboxylate, then add 10ml of anhydrous tetrahydrofuran, and stir for 30min , slowly add 1.23ml of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com