Metal organic framework-calcium carbonate composite crystal material and preparation method thereof
A metal-organic framework and composite crystal technology, applied in the field of nanocomposite materials, can solve problems such as interface incompatibility, and achieve the effect of improving thermal stability and alkali stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
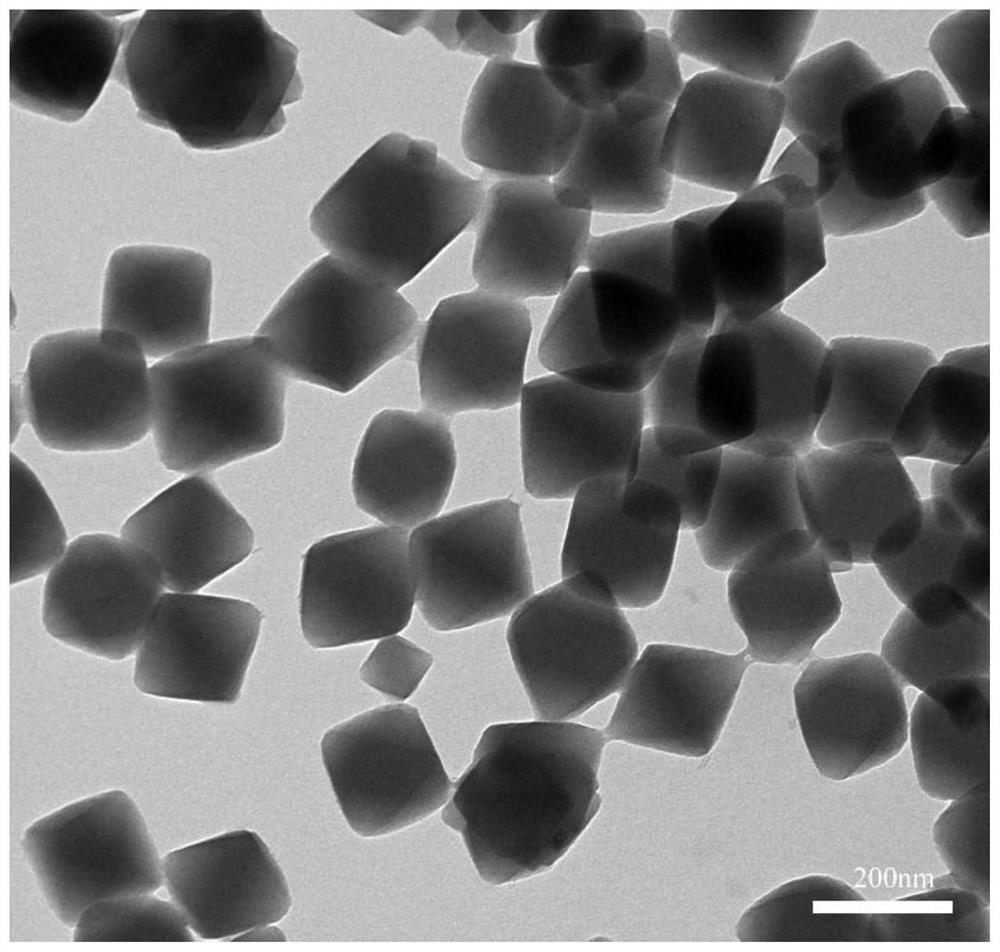
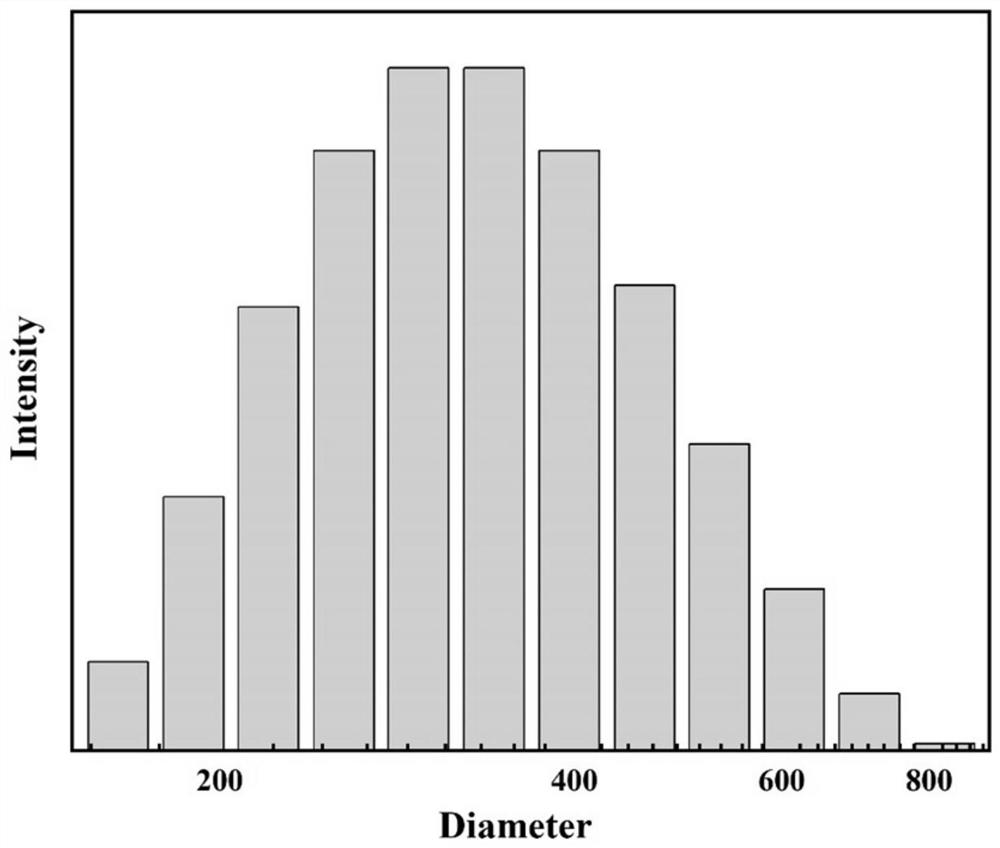
[0050] 0.204g ZrCl 4 Dissolved in 100mL DMF (N,N-dimethylformamide) solvent, 0.29g NH 2 -BDC (2-aminoterephthalic acid) was dissolved in 100mL DMF (N,N-dimethylformamide) solvent, then the two were mixed and 24mL glacial acetic acid was added, reacted in an oil bath at 120°C for 3h, and then Metal-organic frameworks (UiO-66-NH 2 ) crystal particles, the metal organic framework (UiO-66-NH 2 ) transmission electron microscope image of crystal particles figure 1 , the metal-organic framework (UiO-66-NH 2 ) The size (diameter) distribution of crystal particles is as follows figure 2 , it can be seen that the metal organic framework (UiO-66-NH 2 ) The diameter of the crystal particles is between 100-800nm;
[0051] Then, take 0.5g UiO-66-NH 2 , disperse it in 80mL DMF solvent, then add 0.448g of chain transfer agent CPCP (4-cyano-4-(phenylthioformylthio)pentanoic acid), 0.238g of HOBt (1-hydroxy Benzotriazole) and 0.368g of EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimid...
Embodiment 2
[0055] 0.15g ZrCl 4 Dissolved in 100mL DMF (N,N-dimethylformamide) solvent, 0.35gNH 2 -BDC (2-aminoterephthalic acid) was dissolved in 100mL DMF (N,N-dimethylformamide) solvent, then the two were mixed and 20mL formic acid was added, reacted in an oil bath at 80°C for 2h, and then used DMF (N,N-dimethylformamide) was centrifuged to obtain a metal-organic framework (UiO-66-NH 2 ) crystal particles;
[0056] Then, take 0.0625g UiO-66-NH 2 , disperse it in 10mL DMF solvent, then add 0.056g of chain transfer agent CPCP (4-cyano-4-(phenylthioformylthio)pentanoic acid), 0.02976g of HOBt (1-hydroxy Benzotriazole) and 0.046g of EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride), then reacted in an oil bath at 100°C for 24h, after the reaction was completed UiO-66-NH surface modified by chain transfer agent was obtained by centrifuging with methanol at 3000rpm 2 crystal particles; then, UiO-66-NH modified by chain transfer agent 2 Disperse crystal particles into 10...
Embodiment 3
[0059] 0.25g ZrCl 4 Dissolved in 100mL DMF (N,N-dimethylformamide) solvent, 0.25gNH 2 -BDC (2-aminoterephthalic acid) was dissolved in 100mL of methanol solvent, then the two were mixed and 30mL of triethylamine was added, reacted in an oil bath at 30°C for 4h, and then centrifuged with methanol solvent to obtain a metal organic framework (UiO -66-NH 2 ) crystal particles;
[0060] Then, take 0.0625g UiO-66-NH 2 , disperse it in 10mL of methanol solvent, then add 0.112g of chain transfer agent CPCP (4-cyano-4-(phenylthioformylthio)pentanoic acid), 60mg0.06g of HOBt (1- Hydroxybenzotriazole) and 92mg0.092g of EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride), then reacted in an oil bath at 120°C for 24h, and the reaction After completion, use methanol to centrifuge at 3500rpm to obtain UiO-66-NH surface modified by chain transfer agent 2 crystal particles; then, UiO-66-NH modified by chain transfer agent 2 Disperse the crystal particles into 10mL of methan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com