Pichia guilliermondii uricase mutant
A mutant, uricase technology, applied in the field of enzyme biology, can solve the problem of low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Construction of recombinant expression vectors of Pichia mongoliana uricase series mutants
[0037] Select the pseudo-mutation site, entrust Beijing Zhongmei Taihe Biotechnology Co., Ltd. to synthesize the coding gene, construct an expression vector, transform Escherichia coli BL21 (DE3), and confirm the inserted coding sequence by picking a single clone on a conventional kanamycin plate.
Embodiment 2
[0038] Example 2: Expression and purification of uricase mutants from Pichia pastoris
[0039] Transformed E. coli cells were cultured in LB medium (containing 0.1g / L kanamycin) for 4-6h, added 0.24g / mL IPTG and induced at 16°C for 18h, centrifuged at 8000rpm at 4°C for 10min to collect cells, 0.1M Tris The cells were resuspended in -HCl (pH 8.0) buffer solution, ultrasonically disrupted, and centrifuged at 12,000 rpm for 10 min at 4°C to collect the supernatant as a crude sample of soluble uricase.
[0040] DEAE-cellulose chromatography purification: (1) equilibration: equilibrate with 10 times the column volume of 0.1M Tris-HCl (pH 8.0) buffer; (2) loading: the crude sample is loaded at a flow rate of 1.0mL / min; (3 ) elution: directly collect the enzyme activity fraction in the unadsorbed effluent; (4) repeat: repeat the above-mentioned ion exchange column equilibration, sample loading, and elution process to remove as much impurity protein as possible.
Embodiment 3
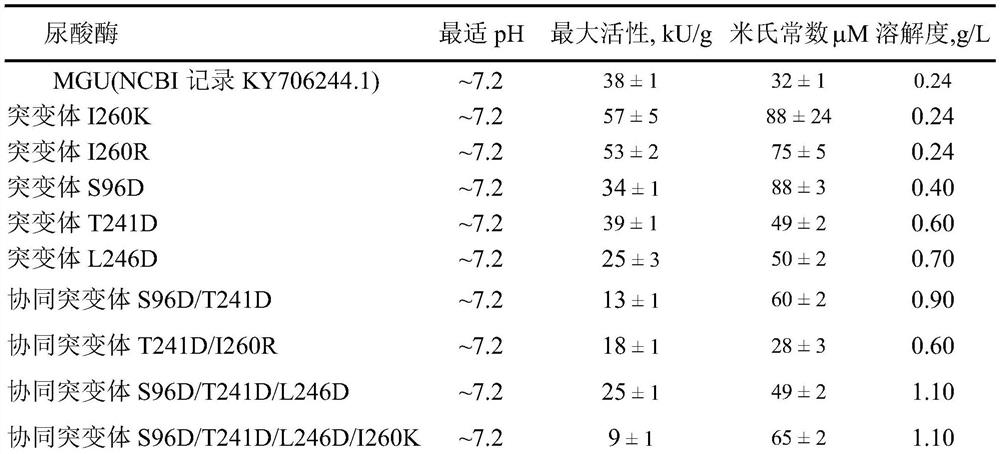
[0041] Example 3: Comparative analysis of activity, optimum pH and solubility of Bacillus fastidiosa uricase series mutants
[0042] (1) Definition of activity measurement: the amount of enzyme required to oxidize 1 μmol of substrate uric acid per minute is an activity unit.
[0043] Enzyme activity measurement steps: use 0.2M borax buffer solution (pH 9.2), measure the uric acid concentration in the system to 75 μmol, delay for 30 s, time interval 10 s, and measure the initial speed of absorption change within 1 minute at 293 nm. The corresponding buffer and uric acid were preheated to (25±0.5)°C, and the extinction coefficient of uric acid was fixed at 11.5 (mmol L -1 cm) -1 .
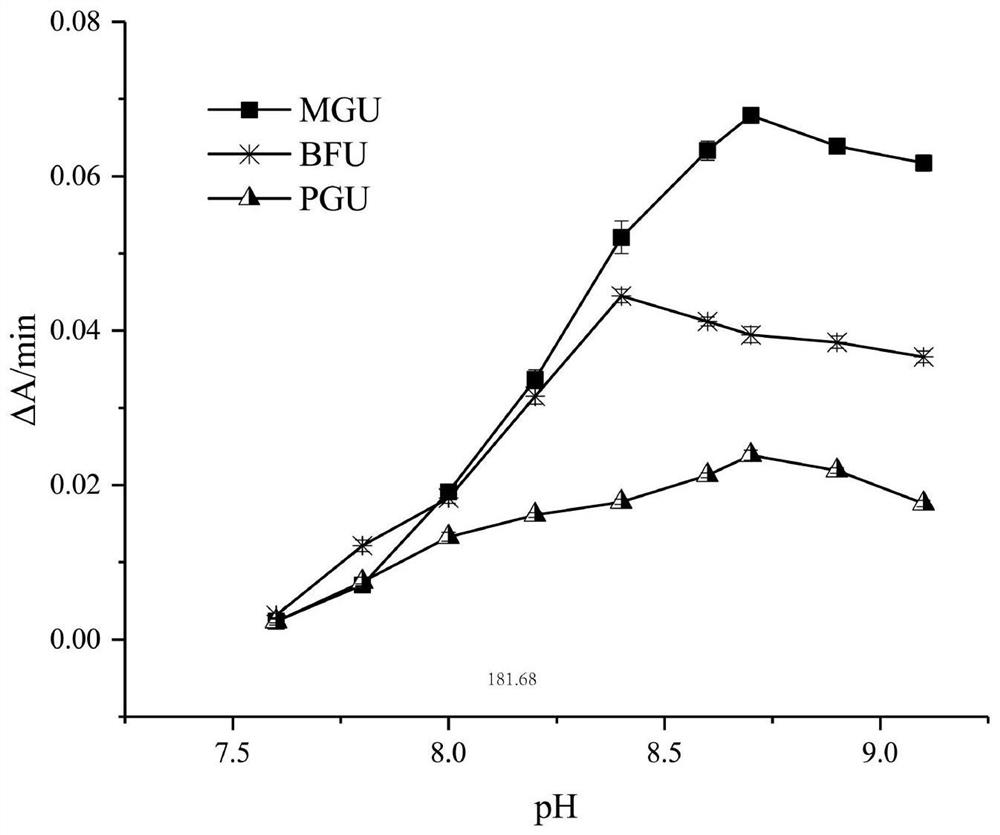
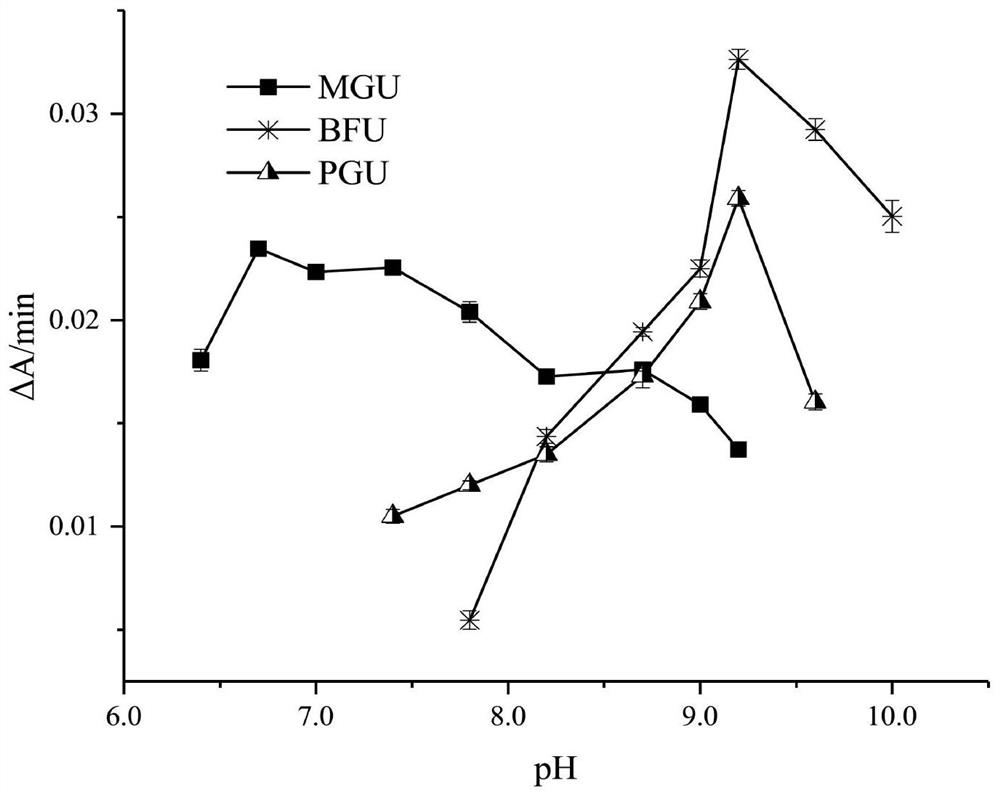
[0044] (2) Determination of optimum pH Select two buffer systems: 0.10M Tris-HCl, pH from 7.4 to 9.2; mix 0.10M borax and boric acid in different proportions to measure pH. The same amount of enzyme was used to measure the change of enzyme activity with pH; the results are shown in Figure 1.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com