A kind of method that solid catalyst catalyzes n-butyraldehyde one-step synthetic isooctylaldehyde
A technology of solid catalyst and n-butyraldehyde, which is applied in the field of green chemistry, can solve problems such as low conversion rate of n-butyraldehyde, decline, and poor catalyst stability, and achieve the effects of broad industrialization prospects, shortened process flow, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Pd / Fe 2 o 3 The preparation process of @C multifunctional catalyst: (1) weigh 32.32g (0.08mol) Fe(NO 3 ) 3 9H 2 O, 2.52g (0.012mol) trimesic acid, 0.035g (0.2mmol) PdCl 2 Mix well with 200mL absolute ethanol; (2) Add the prepared mixed solution into a polytetrafluoroethylene-lined crystallization kettle, raise the temperature to 160°C and keep it for 12 hours; (3) After the reaction, centrifuge and separate dried, and the resulting solid was 2 Calcined at 450°C for 5 hours in the atmosphere to prepare PdO / Fe 2 o 3 @C; (4) PdO / Fe 2 o 3 @C on H 2 Reduction at 300°C for 4 hours in the atmosphere to finally produce Pd / Fe 2 o 3 @C multifunctional catalyst, wherein, the mass fraction of metal Pd is 0.25%, Fe 2 o 3 The mass fraction of is 85%, and the rest is C.
Embodiment 2
[0041] Pd / ZnO-Al 2 o 3 Preparation process of @C multifunctional catalyst: (1) Preparation of ZnO-Al by co-precipitation method 2 o 3 Carrier (Zn / Al molar ratio is 3:1); (2) weigh 4.75g ZnO-Al 2 o 3 Carrier, 0.025g PdCl 2And 0.85g glucose, join in the mixed solution of ethanol and water (10mL, the volume ratio of ethanol and water is 1:1); (3) remove water and ethanol by rotary evaporation at 80 ℃, gained solid is in N 2 Calcined at 500°C for 4 hours in the atmosphere to obtain PdO / ZnO-Al 2 o 3 @C; (4) put the roasted sample in H 2 Pd / ZnO-Al can be prepared by reducing at 350°C for 4 hours in the atmosphere 2 o 3 @C multifunctional catalyst, wherein the mass fraction of metal Pd is 0.3%, ZnO-Al 2 o 3 The mass fraction of is 93.3%, and the rest is C.
[0042] The multifunctional catalysts used in the following reaction examples are all prepared by catalyst preparation example 1, example 2, or similar methods.
Embodiment 3
[0044] Add 30g of n-butyraldehyde and Pd / Fe equivalent to 10% of the quality of n-butyraldehyde in the 100mL autoclave 2 o 3 @C Catalyst, use N first 2 Displacing the air, followed by H 2 Replacement, at a reaction temperature of 160°C, filled with H 2 Maintain the pressure at 2.0MPa, and magnetically stir for 6h. After the reaction, the product liquid was analyzed by gas chromatography, the conversion rate of n-butyraldehyde was 94.3%, and the selectivity of isooctylaldehyde was 96.0%.
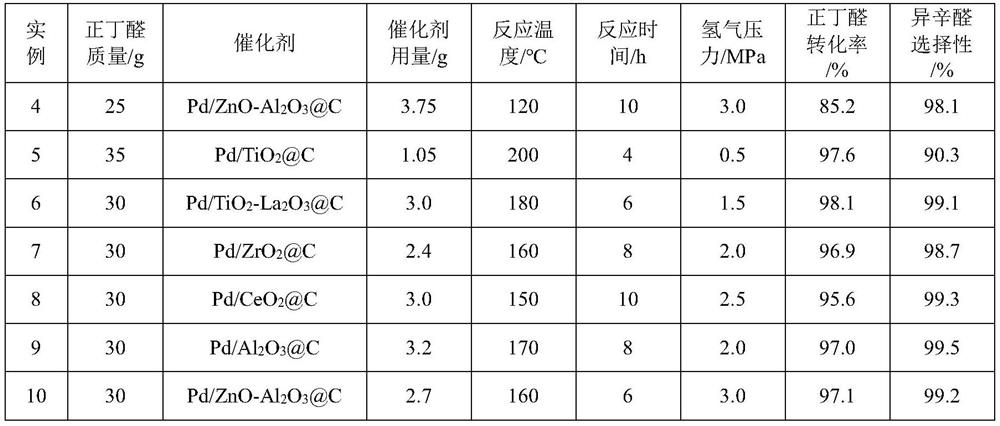
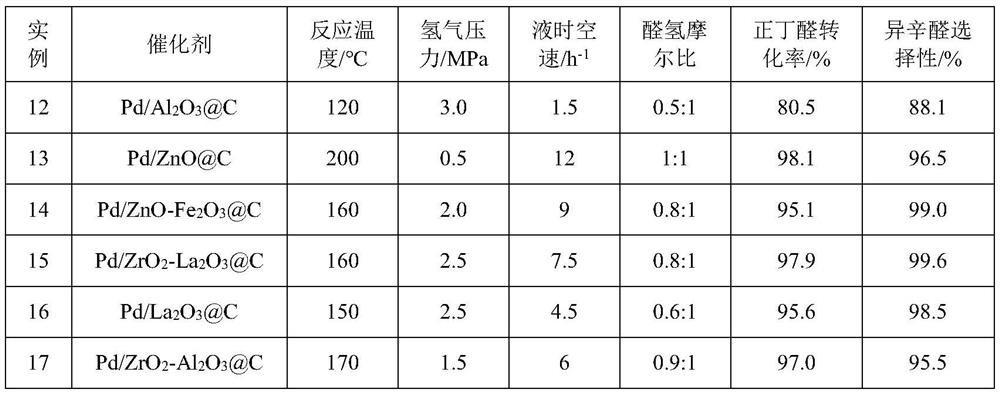
[0045] Examples 4-10 are in accordance with the operation steps of Reaction Example 3, and the reaction conditions and results are shown in the summary table.
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com