Anti-helicobacter pylori recombinant antibody, preparation method and application
A Helicobacter pylori and recombinant antibody technology, which is applied in the field of antibody engineering and biomedicine, can solve the problems of low stability and cumbersome Fab antibody preparation process, and achieve the effect of small molecular weight, easy operation and strong penetrability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Culture against Helicobacter pylori
[0031] A. Helicobacter pylori medium: Weigh 39g of Columbia agar and add it to 1L double distilled water, sterilize at 121°C for 20min, cool to 50°C, add incubating defiberized sheep blood and Helicobacter pylori selective supplement respectively The final concentrations were 7% and 4%.
[0032] B. Take the cryopreserved Helicobacter pylori and immediately put it in a 37°C water bath for 30s, take 100μL and spread it on the Columbia selective medium, and put it in CO 2 Incubator, 37°C (O 2 5%, CO 2 10%, N 2 85%) activated for 24h. After two activations, they were transferred to the newly configured Columbia selective medium and cultured for 12 hours.
Embodiment 2
[0034] Screening Anti-Helicobacter Pylori Single Chain Antibody Using Phage Display Antibody Library Technology
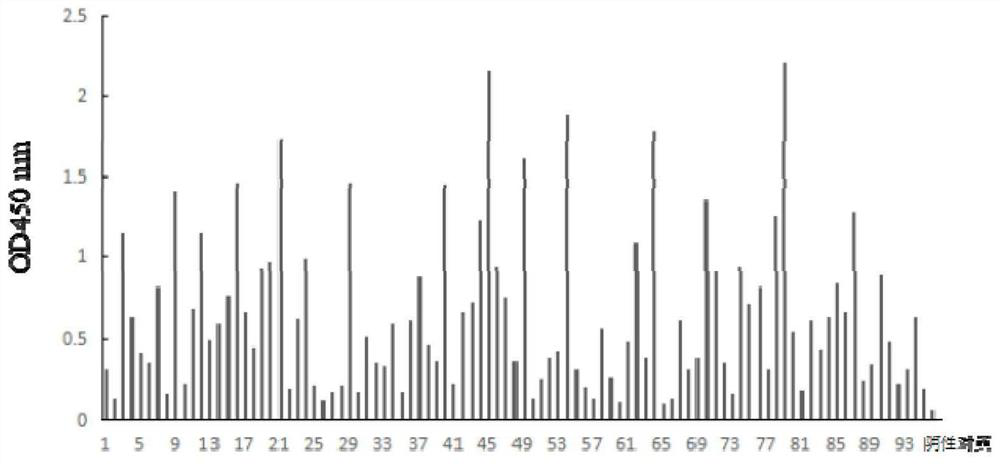
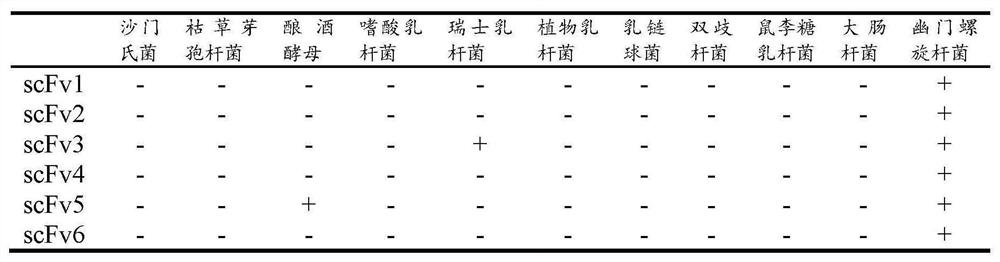
[0035] Our laboratory has constructed a natural fully human scFv antibody library in the early stage, with a library capacity of 2.5×10 8 , good diversity. Using Helicobacter pylori as antigen, the natural fully human scFv antibody library was enriched by phage display by immunomagnetic bead method, clones were randomly selected from the enriched scFv antibody library, and monoclonal phage amplification and expression were performed. The expressed scFv was detected by phage ELISA with anti-M13-HRP monoclonal antibody. The concrete experiment of this embodiment is as follows:
[0036] (1) Add 1.5 mL of 5% MPBS to the EP tube to block overnight, then discard the MPBS and wash with PBS for 3 times.
[0037] (2) Take 100μL concentration to be 1.72×10 13 The CFU phage single-chain antibody library and 200 μL of 5% MPBS were added to the sealed EP tube, mixed evenly,...
Embodiment 3
[0052] ELISA detection of screening results
[0053] (1) The Helicobacter pylori cultured in step B (Example 2) was scraped into a 1.5mL EP tube containing 1mL PBS buffer, mixed evenly, and washed 3 times repeatedly.
[0054] (2) Add 100 μL of 0.25% glutaraldehyde to each well, incubate at 37° C. for 2 hours, and fix the cells.
[0055] (3) Wash each well with 200 μL of PBS to remove unfixed helical rods and excess glutaraldehyde, and wash repeatedly 5 times.
[0056] (4) Add 300 μL of 2% skimmed milk to each well to block the plate, and incubate at 37° C. for 2 hours; wash off excess skimmed milk with 200 μL of PBS, and wash 5 times repeatedly.
[0057] (5) Add 200 μL skimmed milk to each well, and add 10 μL KM13-rescued phage with scFv monoclonal to each well, shake and mix well, and float and incubate at 37° C. for 1 hour.
[0058] (6) Each well was repeatedly washed 5 times with 200 μL of 0.1% Tween20 PBS to remove non-specifically bound phages.
[0059] (7) Add 200 μL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com