Synergistic and depletion-resistant chimeric antigen receptor T cell and application thereof in preparation of medicine for treating tumors
A technology of chimeric antigen receptors and antigens, applied in the direction of antibody medical components, genetically modified cells, antibody mimics/scaffolds, etc., can solve the problems of B cell function loss, tumor recurrence, poor persistence, etc., and achieve a strong immune response , good anti-tumor, strong anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1. Construction of a CAR lentivirus containing five elements of DAP12-4-1BB-T2A-CD19(scFv)-KIRS2
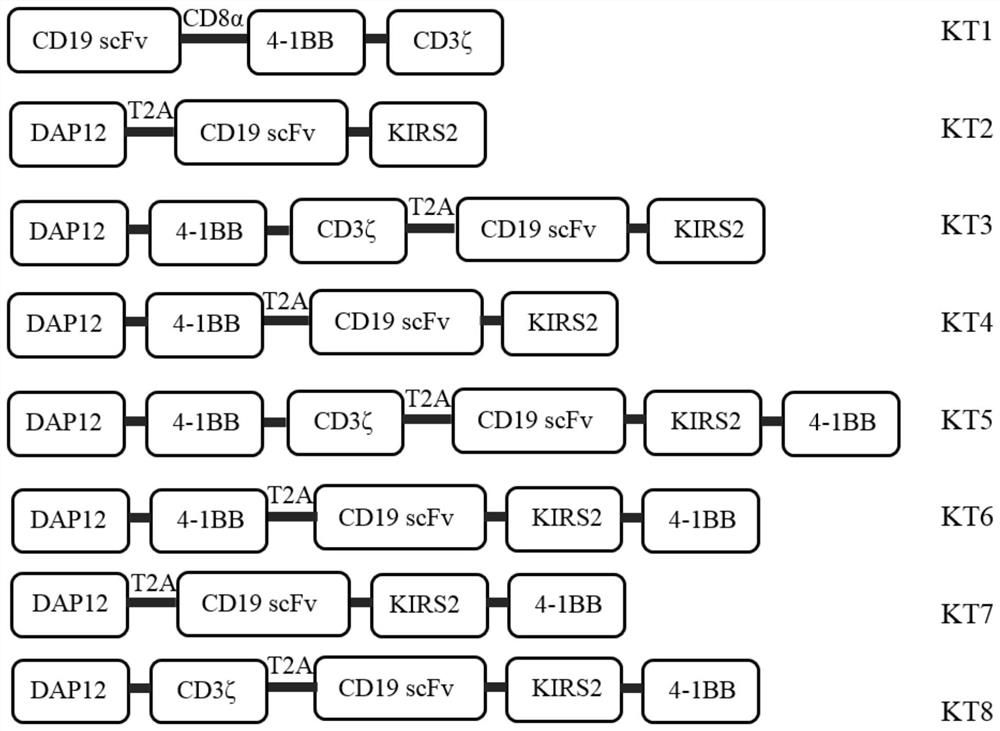
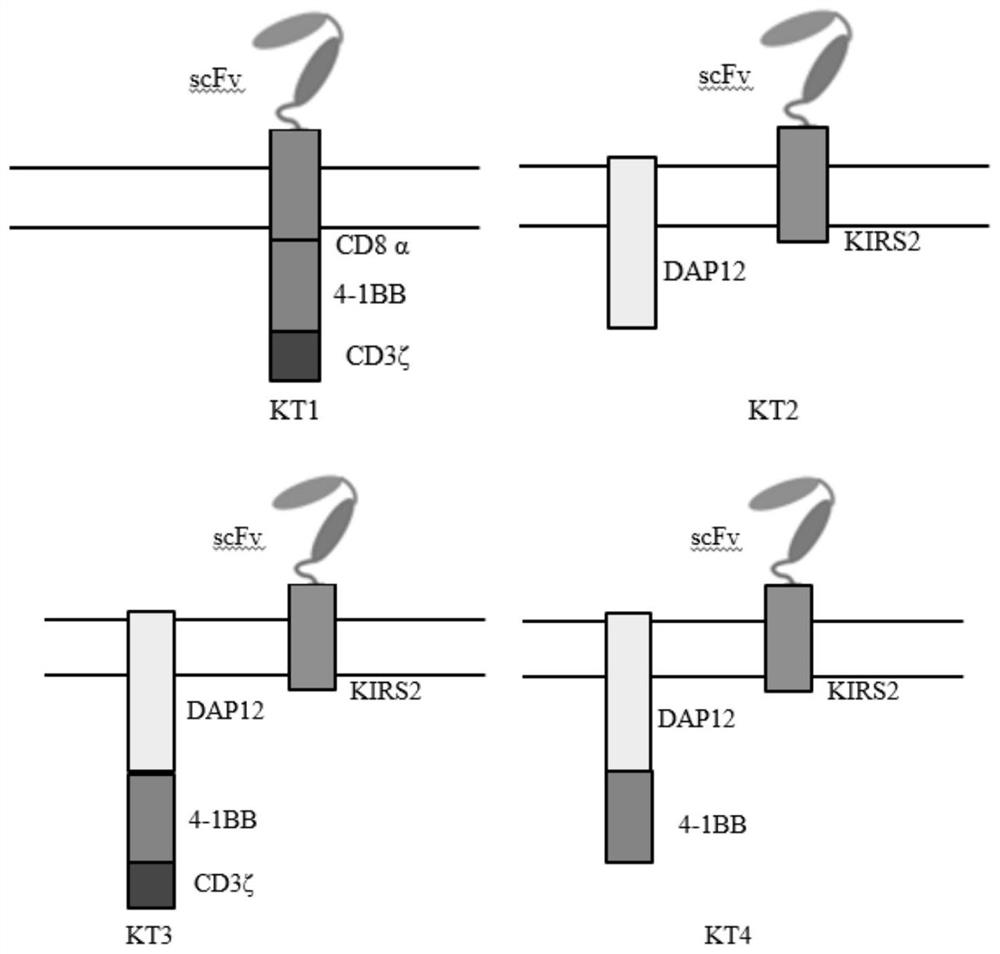
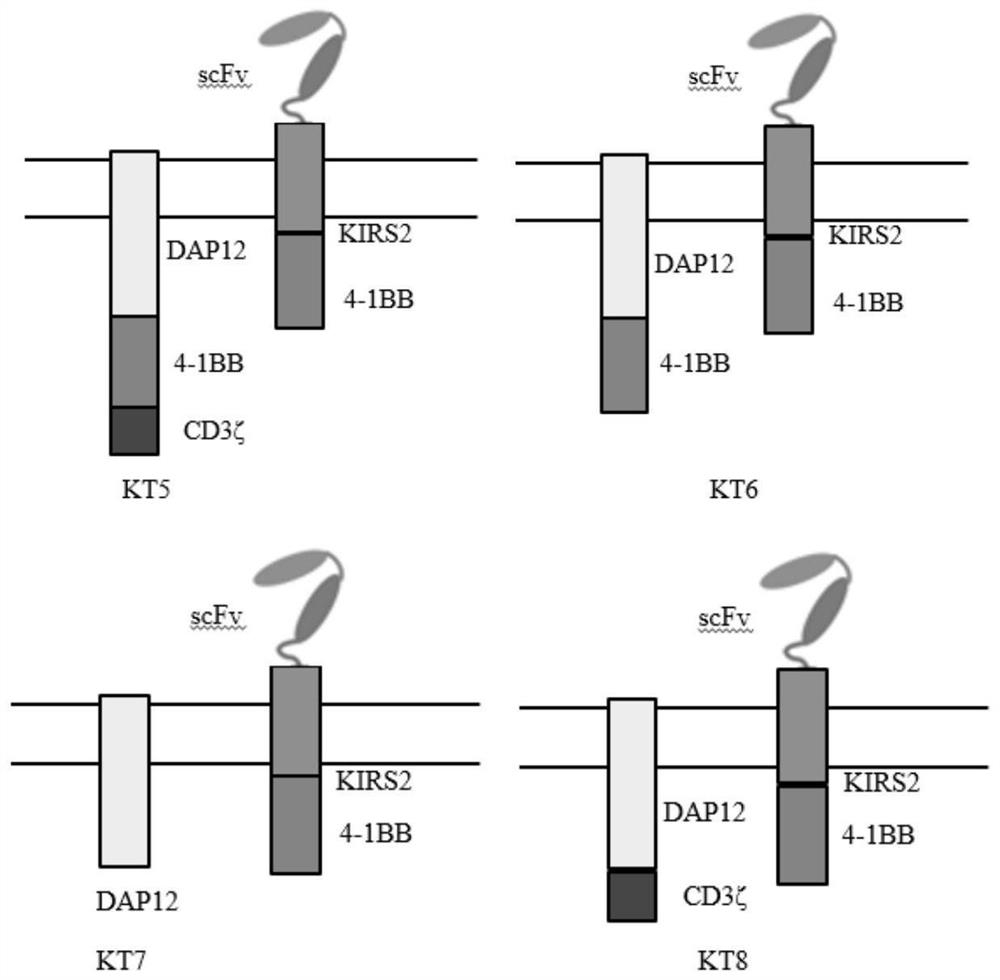
[0053] In order to prove that CAR-T cells containing DAP12-4-1BB-KIRS2 intracellular signaling domain contain 4-1BB-CD3ζ, DAP12-KIRS2, DAP12-4-1BB-CD3ζ-KIRS2-4-1BB, DAP12 -41BB-KIRS2-4-1BB, DAP12-KIRS2-4-1BB, and DAP12-CD3ζ-KIRS2-4-1BB stimulated CAR-T cells have more advantages, so it is necessary to construct viral vectors containing different combinations of stimulating signals. In this example, the single-chain antibody targeting B-cell malignancies (CD19) is used as a unified extracellular antigen recognition structure, and the following eight chimeric antigen receptors need to be constructed respectively ( figure 1 and figure 2 ):
[0054] CD19(scFv)-CD8α-4-1BB-CD3ζ (KT1)
[0055] DAP12-T2A-CD19(scFv)-KIRS2 (KT2)
[0056] DAP12-4-1BB-CD3ζ-T2A-CD19(scFv)-KIRS2 (KT3)
[0057] DAP12-4-1BB-T2A-CD19(scFv)-KIRS2 (KT4)
[0058] DAP12-4-1BB-CD3ζ-T2A-CD19(scFv)...
Embodiment 2
[0103] Embodiment 2, virus infection T cell
[0104] 1. Isolation and activation of T cells and virus infection
[0105] (1) Isolation of human peripheral blood mononuclear cells
[0106] About 10 ml of peripheral blood was collected with a blood collection tube containing an anticoagulant, centrifuged at 3000 rpm for 30 min, and the upper layer plasma was collected, and the collected upper layer plasma was centrifuged at 5000 rpm for 10 min. Add it to the lymphocyte separation medium (purchased from Tianjin Haoyang Biological Products Technology Co., Ltd.) at a volume ratio of 1:1, gradient centrifugation, 3000rpm, and centrifugation for 30min. After centrifugation, the centrifuge tubes are layered from top to bottom: the first The first layer is the plasma layer; the second layer is the buffy coat layer of lymphocytes; the third layer is the transparent separation liquid layer; the fourth layer is the red blood cell layer. Aspirate the buffy coat of lymphocytes, wash twice...
Embodiment 3
[0111] Example 3. In vitro killing effect evaluation of virus-infected CAR-T cells
[0112] (1) Culture target cells MCF7-CD19 cells (CD19 positive cell line), 293T (CD19 negative cell line) and effector cells KT1, KT2, KT3, KT4, KT5, KT6, KT7, KT8, a total of 8 groups of CAR-T cells ;
[0113] (2) Collect target cells and effector cells, centrifuge at 1500rpm for 5min, and discard the supernatant;
[0114] (3) Resuspend target cells and effector cells with 10% FBS+1640 complete medium;
[0115] (4) Using the real-time cell analysis system (RTCA), add 50 μL of 1640 medium to the well of E-Plate16
[0116] (5) Use RTCA to detect the baseline, and confirm that the contact of the selected well is normal;
[0117] (6) Set the effect-target ratio to 0:1, 1:1, 5:1, 10:1;
[0118] (7) Take out E-Plate16, add 50 μL of uniformly mixed target cell suspension into each well according to the effect-to-target ratio, so that the number of cells in each well is 10 4 cells / 50μL;
[0119...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com