High performance liquid chromatography analysis method of stannous sodium glucoheptonate related substances for injection
A technology of high performance liquid chromatography and sodium glucoheptonate, applied in the field of drug analysis, can solve the problem of no related substance check items, etc., and achieve the effects of being conducive to safe promotion and application, rapid and accurate detection and monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Preparation of solvent: water: acetonitrile = 50:50 (v / v).
[0049] Preparation of blank solution: water: acetonitrile = 50:50 (v / v).
[0050] Preparation of blank auxiliary material solution: Weigh 20 mg of urea, add 10 mL of solvent precisely, shake well, and obtain blank auxiliary material solution.
[0051] Preparation of the test solution: Weigh 160 mg of sodium glucoheptonate, 200 mg of urea and 1.6 mg of stannous chloride, add 10 mL of solvent precisely, and shake well to obtain the test solution.
[0052] Preparation of the contrast solution: Accurately measure 0.5 mL of the test solution, put it in a 50 mL measuring bottle, dilute with a solvent to the volume, shake well, and obtain the contrast solution.
[0053] Preparation of sodium glucoheptonate positioning solution: weigh 16 mg of sodium glucoheptonate, accurately add 1 mL of solvent, and shake well to obtain.
[0054] Preparation of biuret positioning solution: take 1 mg of biuret reference substance, ...
Embodiment 1
[0069] Example 1: System Adaptability
[0070] Prepare solvent and control solution according to the analysis method, and carry out chromatographic test according to the analysis method. After the baseline is balanced, take 1 injection of solvent, take 3 consecutive injections of control solution, record the chromatogram and the theoretical plate number of the first injection of sodium glucoheptonate in the control solution, and calculate the peak of 3 injections of sodium glucoheptonate RSD of the area.
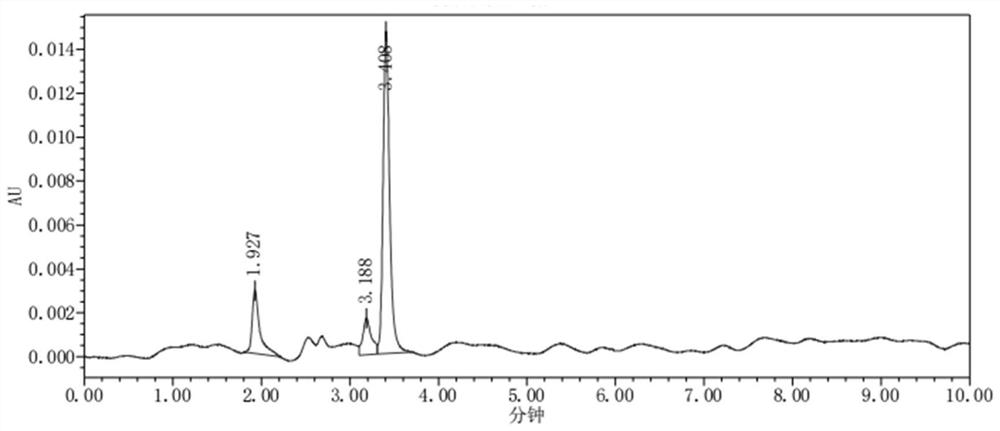
[0071] The requirements of various measurement standards are: the number of theoretical plates of the first injection of sodium glucoheptonate in the control solution shall not be less than 3000, and the RSD of the peak area of sodium glucoheptonate in repeated injections of the control solution for three injections shall not be greater than 4.0%. The chromatogram of the first injection of the control solution is shown in figure 1 .
[0072] Table 2 Results of system ad...
Embodiment 2
[0075] Example 2: Specificity
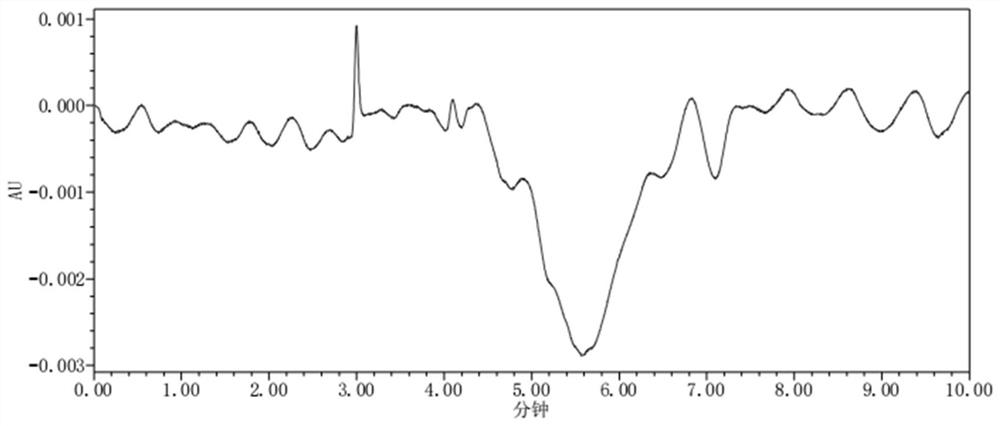
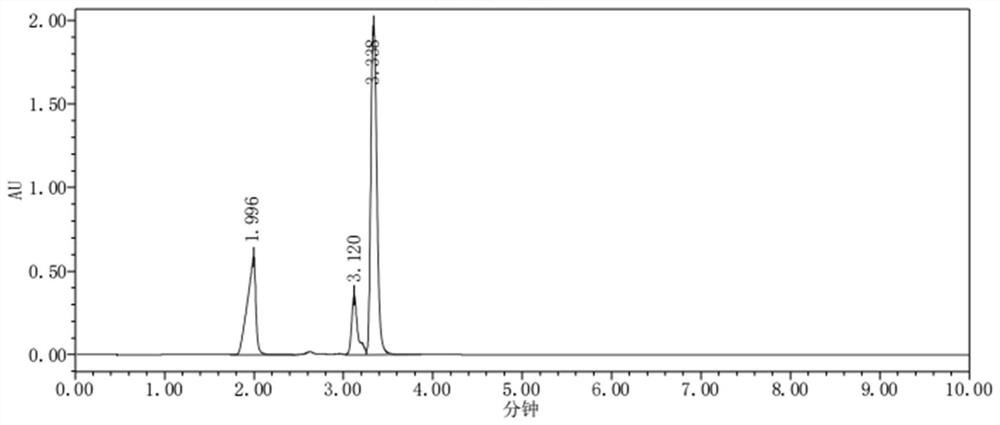
[0076] Take the blank solution, blank excipient solution, test solution, sodium glucoheptonate positioning solution, and biuret positioning solution, and test according to the chromatographic conditions under "Analytical Method", inject 1 needle each, and record the chromatogram. For the chromatogram of the blank solution, see figure 2 ; The chromatogram of the test solution is shown in image 3 ; The chromatogram of sodium glucoheptonate positioning solution is shown in Figure 4 ; Biuret positioning solution chromatogram see Figure 5 .
[0077] The requirements of various measurement standards are: at the retention time of the test solution sodium glucoheptonate, the interference of the blank solution and the blank auxiliary material solution is ≤0.1%. In the chromatogram of the test solution, the separation between the sodium glucoheptonate peak and the adjacent impurity peaks is not less than 1.5.
[0078] At the corresponding retenti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com