Method for preparing pyrrolidone and derivatives thereof by continuous tubular reactor
A tubular reactor, pyrrolidone technology, applied in the direction of organic chemistry, etc., can solve the problems of low utilization rate of equipment, difficult to improve production efficiency, complicated process, etc., to improve production efficiency, realize automation and continuity, and simple operation mode Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
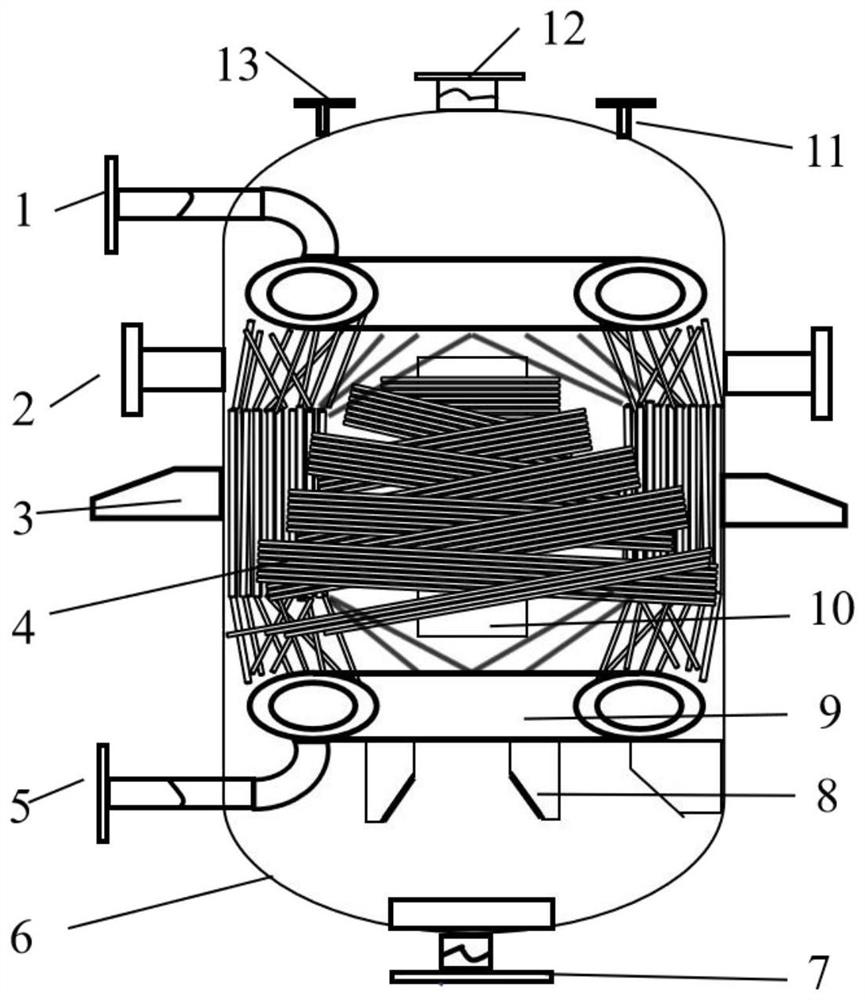
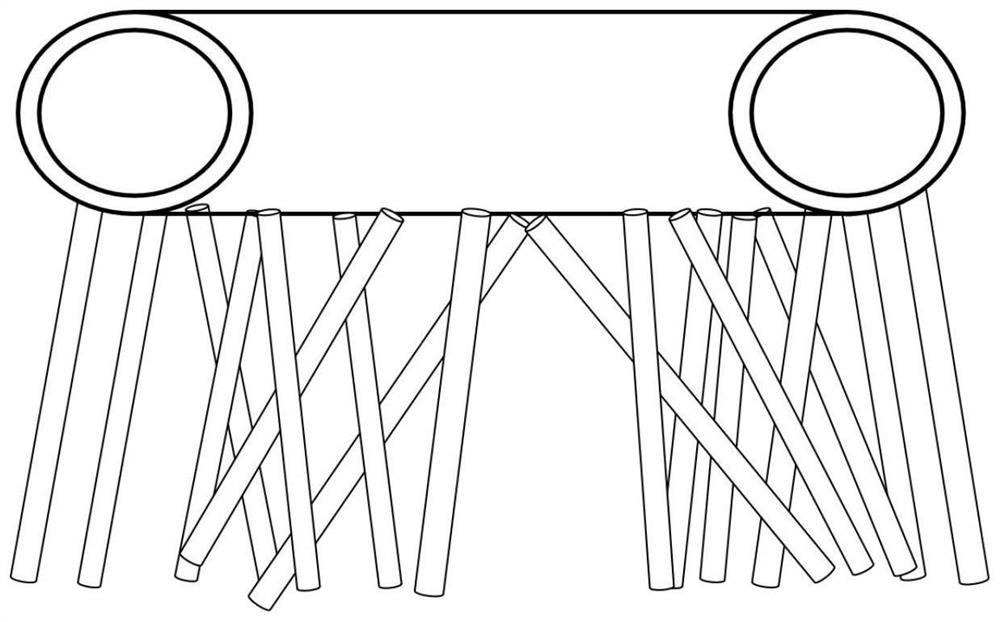
[0025] After mixing and stirring γ-butyrolactone, ethanolamine and solvent water, they are passed into a vertical winding tube reactor. When the reaction temperature is 270°C and the pressure is 7.7MPa, the residence time of the reaction materials in the reaction section is 4.3h , the molar ratio between γ-butyrolactone and ethanolamine is 1.08, and the amount of solvent is 1.2 times the mass of γ-butyrolactone; the reacted material is extracted from the lower end of the vertical winding tube reactor, and will be collected The obtained sample is rectified by a rectification tower and analyzed by gas chromatography to obtain N-hydroxyethylpyrrolidone; the reaction output is rectified to recover and recycle excess γ-butyrolactone.
Embodiment 2-4
[0027] Other steps are the same as in Example 1, except that the molar ratio of γ-butyrolactone:ethanolamine is changed.
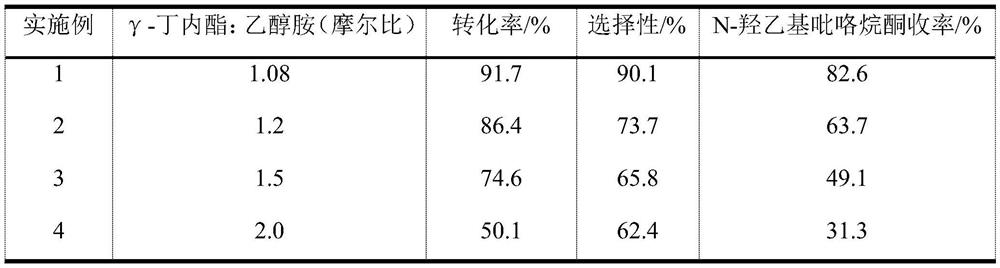
[0028] The partial result of embodiment 1-4 reaction sees the following table:
[0029]
Embodiment 5-8
[0031] Other steps are the same as in Example 1, the difference is that the temperature is 275°C, the pressure is 8.0MPa, the amount of solvent is 2.2 times the mass of γ-butyrolactone, the residence time of the reaction material in the reaction section is 3.0h, and the γ-butyrolactone is adjusted. The molar ratio between butyrolactone and ethanolamine, the partial result of reaction sees the following table:
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com