Preparation method of blonanserin intermediate
A technology of blonanserin and intermediates, which is applied in the field of medicinal chemistry, can solve the problems of complex products, long reaction time to the end point, and no effect is achieved, and achieves simple reaction steps, stable effective conversion rate, and increased uniformity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
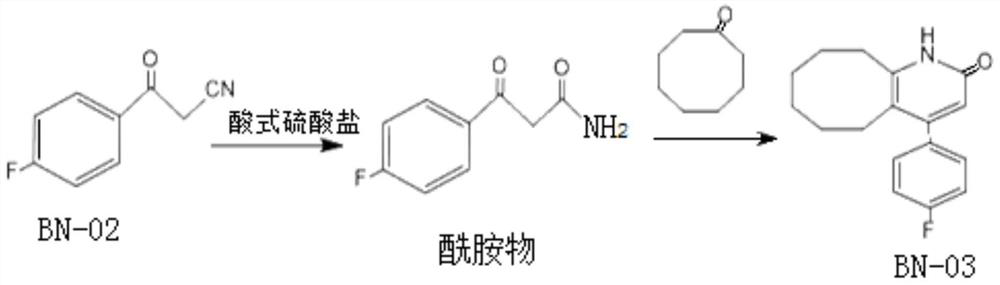
[0038] 1. Preparation 3- (4-fluorophenyl) -3 oxypropamide with sodium hydrogen sulfate
[0039] In a 250 ml of clean reaction flask, 25.0 g (0.15 mol) was added to fluorobenzoyl acetonitrile, 36 ml of water, 36 g sodium hydrogen sulfate (0.3 mol). The reaction mixture was warmed to 60 ° C with rapid stirring, and the temperature was controlled for 3 hours. HPLC monitors the reaction process, when the residual amount of fluorobenzoyloy in the system is less than 1%, it is considered to be complete, reactive, cooling to 30 ° C, dropping the mass concentration of 12%, tonifying pH 9. The controlled temperature was stirred at 15 ° C for 1 hour, filtered, washed, filtered, dried 80 ° C to give 3- (4-fluorophenyl) -3 oxpropanamide 25.5 g, yield 92%, HPLC 98.5%.
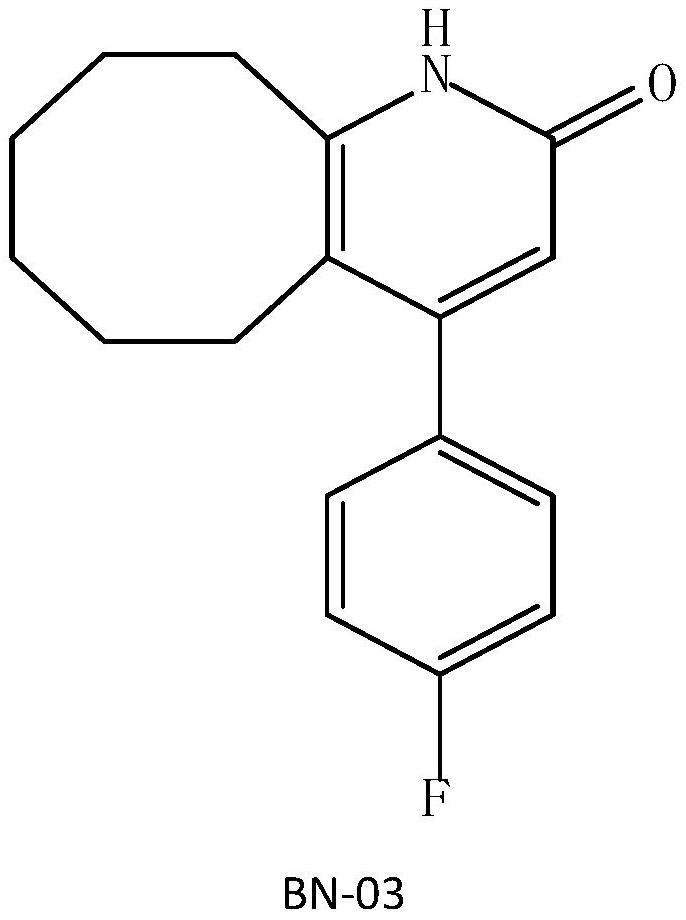
[0040] 2. Preparation of BN-03 (4- (4-fluorophenyl) -5, 6, 7, 8, 9, 10-hexahydrocycloalkane-2 (1H)-ketone) by pyridine catalysis
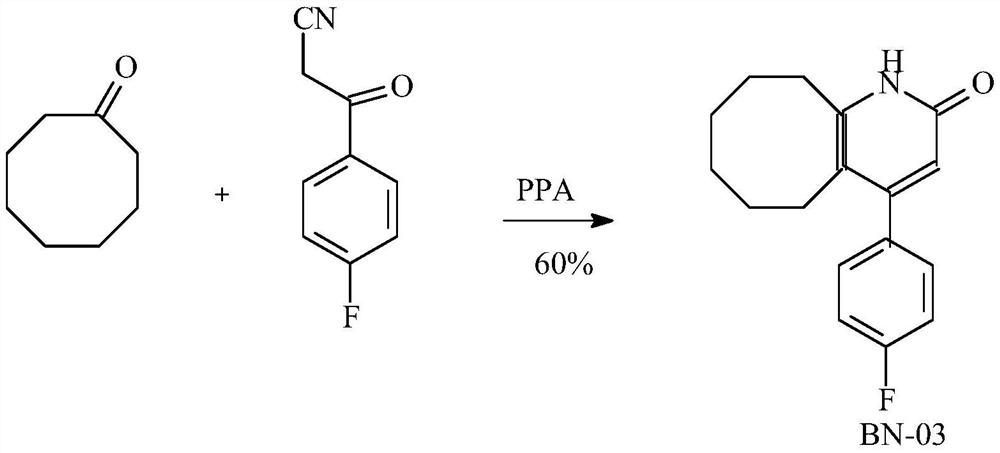
[0041]In a 250 ml of clean reaction flask, 125 g of polyphosphoric acid was sequentially added t...
Embodiment 2
[0043] 1. Preparation of 3- (4-fluorophenyl) -3 oxpropanamide using a catalyst using a catalyst using a zirconium sulfate
[0044] In a 250 ml of clean reaction flask, 25.0 g (0.15 mol) was added to fluorobenzoyloy, 36 ml of water, 85 g of zirconium sulfate (0.3 mol). Other and the step 1 of Example 1 were the same, after drying at 80 ° C, 3- (4-fluorophenyl) -3 oxpropanamide 26.3 g, yield 95%, HPLC 99.2%.
[0045] 2. Preparation of BN-03 (4- (4-fluorophenyl) -5, 6, 7, 8, 9, 10-hexahydrocycloalkane-2 (1H)-ketone) by pyridine catalysis
[0046] In a 250 ml clean reaction bottle, 125 g of polyphosphoric acid was sequentially added, and 3- (4-fluorophenyl) -3 oxypropanamide obtained by step 1, 20.0 g of cyclophily of cyclophily, 2 ml of pyridine. Other and the step 2 of Example 1 were the same, and the white crystalline solid 34 g was obtained. The yield was 91.07%, purity (HPLC): 99.3%, melting point 235.0-236.0 ° C.
Embodiment 3
[0048] 1, 3- (4-fluorophenyl) -3 oxypropanamide was prepared using a catalyst using a catalyst of zirconium sulfate, and step 1 of the second embodiment.
[0049] 2, use DMAP (N, N-dimethylaminopyridine) to prepare BN-03 (4- (4-fluorophenyl) -5,6,7,8,9,10-hex hydrogen octanepyridine -2 (1H) - ketone)
[0050] In a 250 ml of clean reaction flask, 125 g of polyphosphoric acid was sequentially added, 3- (4-fluorophenyl) -3 oxypropanamide 25g, cyclophily of cyclophily, 0.0 g, DMAP 1.85 g (0.02 mol). The increased, heated to 115 ° C, and the temperature was controlled for 4 hours. HPLC monitors the reaction process, which is considered to be completed when the residual amount of 3- (4-fluorophenyl) -3 oxpropanamide is less than 3% in the system. Cooling to 80 ° C, add 120 ml of ethanol, thoroughly stirred, and then droplets into 1200 mL of dilute alkali, stirred alcohol, filtrate, derived white solid, mixed with 200 ml of tert-butyl methyl ether, white crystalline solid 35.5g. The yiel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com