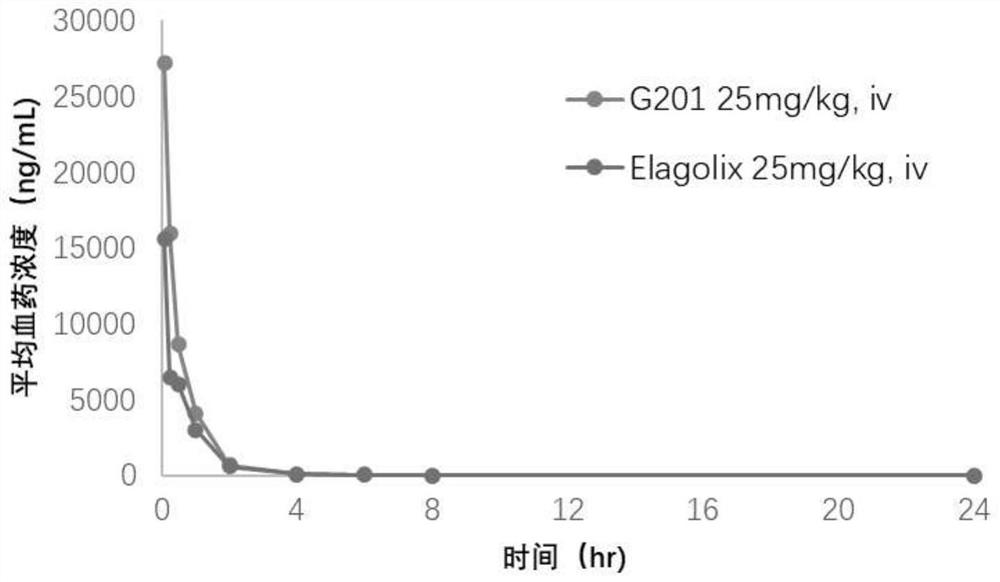
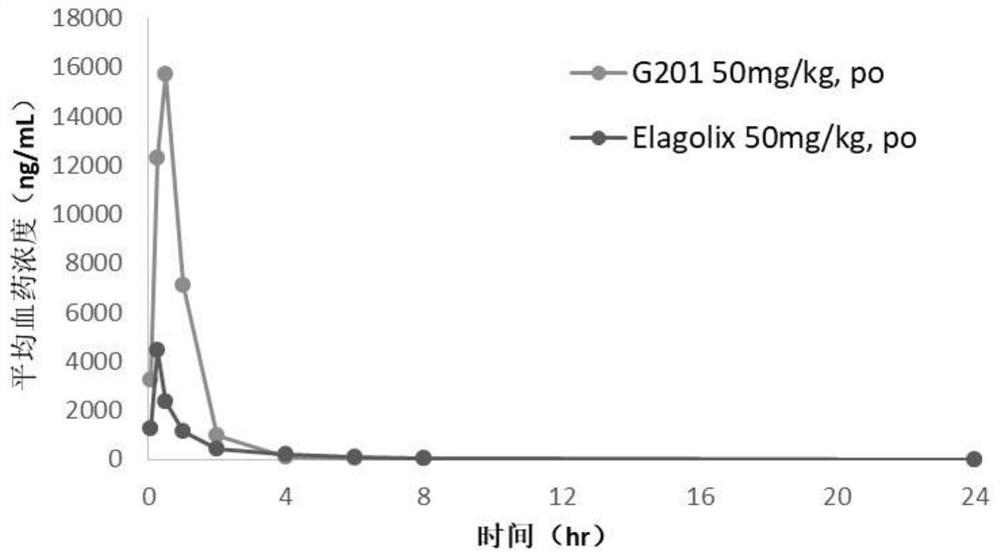
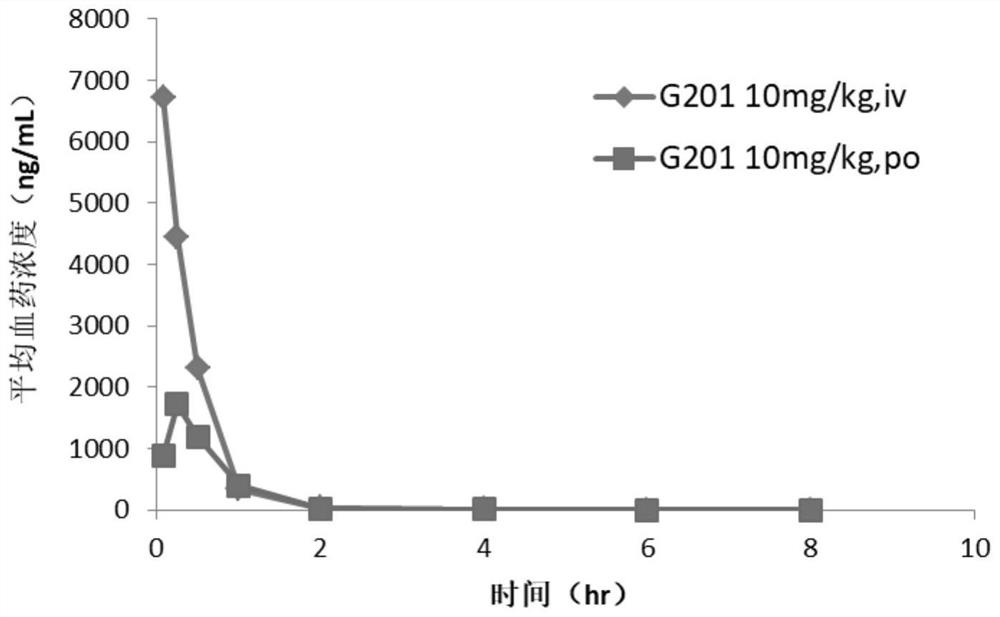
Hormone releasing hormone receptor antagonists and uses thereof
A use, gonadal technology, applied in the direction of diseases, antineoplastic drugs, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The second aspect of the present invention provides a method for preparing the compound provided by the first aspect of the present invention, comprising: hydrolyzing the compound of formula 1-12 to prepare the compound of formula I, and the reaction equation is as follows:
[0038]
[0039] In the preparation method provided by the present invention, the hydrolysis reaction can usually be carried out in the presence of a base. Those skilled in the art can select the appropriate type and amount of alkali for the above-mentioned hydrolysis reaction. For example, the alkali can be alkali metal hydroxide, etc., more specifically lithium hydroxide, etc., and for another example, the alkali The dosage relative to the compound of formula 1-12 is generally equal or in excess. Specifically, the molar ratio of the compound of formula 1-12 to the base can be 1:1.4-1.6.
[0040] In the preparation method provided by the present invention, the reaction can usually be carried out...
Embodiment 1
[0054] The concrete preparation route of compound G201 in the embodiment is as follows:
[0055]
[0056] Compound 1-1 (10 g, 72.4 mmol), pyridine (200 mL) and selenium dioxide (16 g, 144.8 mmol) were added into a three-neck flask. Under the protection of nitrogen, react at 100°C for 2 hours. After the reaction was completed, it was suction filtered, and the filtrate was spin-dried, then 1N hydrochloric acid was added, and extracted with ethyl acetate. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, filtered with suction, and spin-dried to obtain off-white solid 1-2 (12 g).
[0057] Compound 1-2 (12 g, 71.4 mmol) and methanol (200 mL) were added into a three-neck flask. Thionyl chloride (17 g, 142.8 mmol) was added dropwise at 0° C. and stirred overnight at room temperature. After the reaction was completed, methanol was spin-dried, and saturated aqueous sodium bicarbonate solution was added, and extracted with ethyl acetate. The ...
Embodiment 2
[0069] Calcium flux detection:
[0070] FLIPR calcium flux assay kit (Calcium 4 assay kit) was used to measure intracellular calcium changes in recombinant human gonadotropin-releasing hormone receptor (GnRHR) stable cell line CHO-K1 / GNRHR / Gα15. GnRHR is a G protein-coupled receptor (GPCRs). GPCRs signal through the Gq pathway to release intracellular calcium. Therefore, detecting intracellular calcium release through the calcium ion-sensitive fluorescent probe can detect signal transduction through the Gq pathway. Functional changes of GnRHR. The experimental steps are as follows:
[0071] 1. Using F12+10% FBS medium, according to 10000cells / well, 20ul / well, cells were planted in 384-well plates (Corning, Cat#:3764), and cultured overnight (18h).
[0072] 2. Prepare probenecid stock solution (500mM) and Calcium 4 stock solution at a ratio of 1:100 to make dye working solution. Dilute the test product G201 and Elagolix (2mM in DMSO) with assay buffer: Take 5μl of 2mM stock ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com