Preparation method of clarithromycin capsule
A technology for clarithromycin and capsules, which is applied in the field of preparation of clarithromycin capsules, can solve the problems that affect the comprehensive performance of clarithromycin capsules, the percentage of granules are sticky and punched, the difference in capsule filling volume needs to be improved, etc., and achieves low price and easy acquisition. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The present invention provides a kind of preparation method of clarithromycin capsule, comprises the following steps:
[0019] a. Mix clarithromycin and diluent according to the prescription amount, add binder, and stir quickly to make wet granules;
[0020] b. drying the prepared wet granules into dry granules;
[0021] c. The dry granules are sieved and granulated, and mixed with a lubricant;
[0022] d. Determine the content of clarithromycin in the granules to determine the weight of the granules;
[0023] e, filling as capsules.
[0024] The diluent is starch, the binder is starch slurry, and the lubricant is magnesium stearate.
[0025] Further, step a is also included before step a 0 : Sieve starch and magnesium stearate through 100 sieves.
[0026] The drying temperature in the step b is 60°C; in the step c, the dry granules are passed through a 18-mesh sieve for granulation.
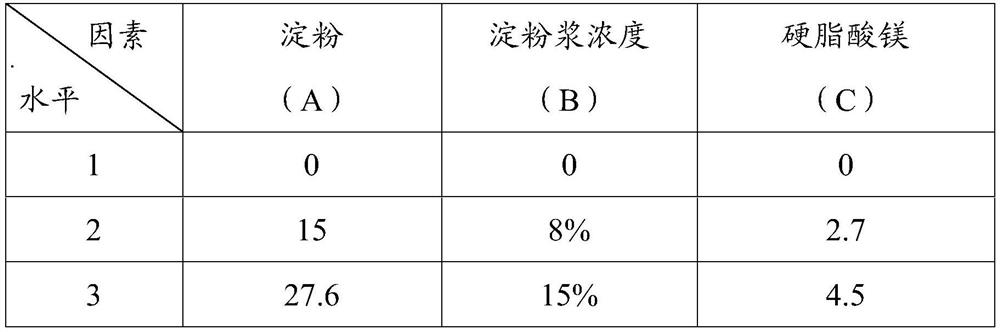
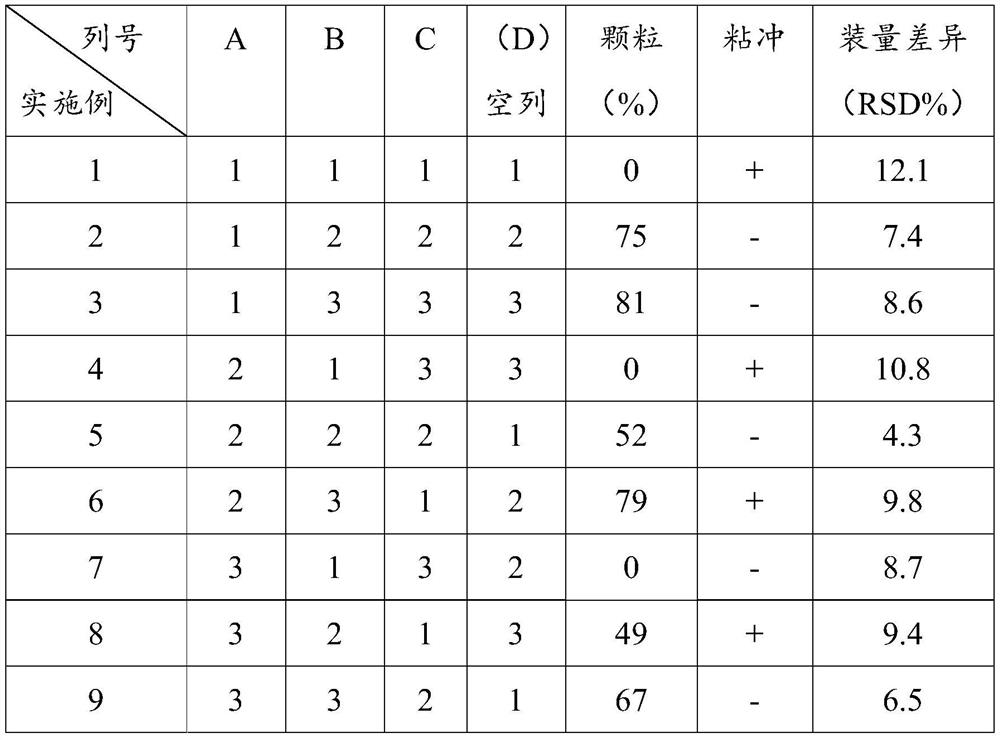
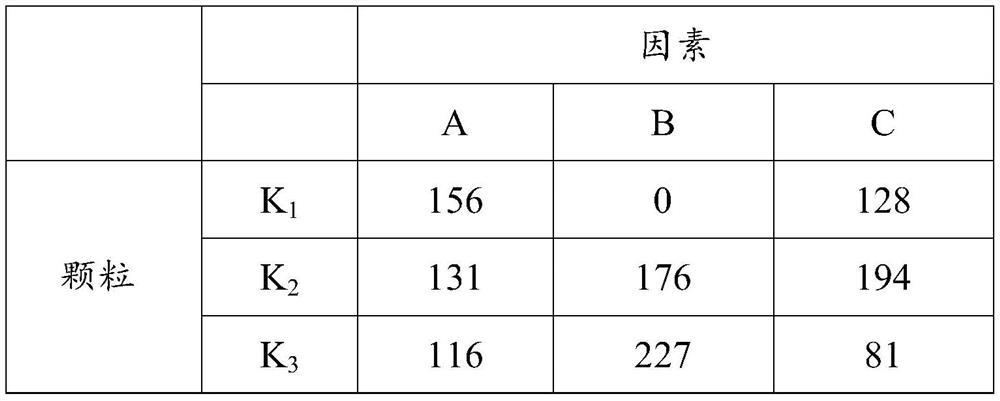
[0027] The embodiment of the present invention utilizes four factors and three ...
Embodiment 1
[0032] In this example, 1000 clarithromycin capsules were prepared, and 250.0 g clarithromycin was directly filled into capsules without adding auxiliary materials (starch, starch slurry concentration, magnesium stearate).
Embodiment 2
[0034] The present embodiment prepares 1000 clarithromycin capsules, the steps are as follows:
[0035] a 0 100 mesh sieves are passed through the magnesium stearate;
[0036] a. Add 250 g of clarithromycin and 50 ml of starch slurry with a concentration of 8% to the wet granulator, stir rapidly for 5 minutes, and prepare wet granules;
[0037] b. Drying the prepared wet granules at 60°C into dry granules;
[0038] c. The dry granules are passed through a 18-mesh sieve for granulation, and 2.7 g of magnesium stearate is added and mixed;
[0039] d. Determine the content of clarithromycin in the granules to determine the weight of the granules;
[0040] e. Capsule filling is carried out with a capsule filling machine, and the empty capsule shell is a No. 0 capsule.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com